Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane
- PMID: 9843581
- PMCID: PMC25656
- DOI: 10.1091/mbc.9.12.3455
Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane
Abstract
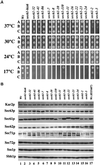
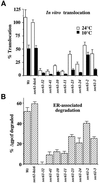
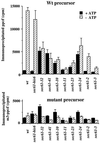
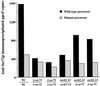
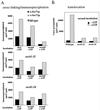
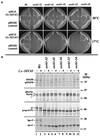
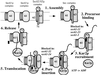
The evolutionarily conserved Sec61 protein complex mediates the translocation of secretory proteins into the endoplasmic reticulum. To investigate the role of Sec61p, which is the main subunit of this complex, we generated recessive, cold-sensitive alleles of sec61 that encode stably expressed proteins with strong defects in translocation. The stage at which posttranslational translocation was blocked was probed by chemical crosslinking of radiolabeled secretory precursors added to membranes isolated from wild-type and mutant strains. Two classes of sec61 mutants were distinguished. The first class of mutants was defective in preprotein docking onto a receptor site of the translocon that included Sec61p itself. The second class of mutants allowed docking of precursors onto the translocon but was defective in the ATP-dependent release of precursors from this site that in wild-type membranes leads to pore insertion and full translocation. Only mutants of the second class were partially suppressed by overexpression of SEC63, which encodes a subunit of the Sec61 holoenzyme complex responsible for positioning Kar2p (yeast BiP) at the translocation channel. These mutants thus define two early stages of translocation that require SEC61 function before precursor protein transfer across the endoplasmic reticulum membrane.
Figures



Similar articles
-
The protein translocation channel mediates glycopeptide export across the endoplasmic reticulum membrane.Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4609-14. doi: 10.1073/pnas.090083497. Proc Natl Acad Sci U S A. 2000. PMID: 10758167 Free PMC article.
-
Sec61p and BiP directly facilitate polypeptide translocation into the ER.Cell. 1992 Apr 17;69(2):353-65. doi: 10.1016/0092-8674(92)90415-9. Cell. 1992. PMID: 1568250
-
SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus.J Biol Chem. 1994 Nov 4;269(44):27478-85. J Biol Chem. 1994. PMID: 7961662
-
Emerging View on the Molecular Functions of Sec62 and Sec63 in Protein Translocation.Int J Mol Sci. 2021 Nov 25;22(23):12757. doi: 10.3390/ijms222312757. Int J Mol Sci. 2021. PMID: 34884562 Free PMC article. Review.
-
Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane.J Cell Sci. 1999 Dec;112 ( Pt 23):4185-91. doi: 10.1242/jcs.112.23.4185. J Cell Sci. 1999. PMID: 10564637 Review.
Cited by
-
Endoplasmic reticulum dynamics, inheritance, and cytoskeletal interactions in budding yeast.Mol Biol Cell. 2002 Mar;13(3):854-65. doi: 10.1091/mbc.01-04-0184. Mol Biol Cell. 2002. PMID: 11907267 Free PMC article.
-
INS-gene mutations: from genetics and beta cell biology to clinical disease.Mol Aspects Med. 2015 Apr;42:3-18. doi: 10.1016/j.mam.2014.12.001. Epub 2014 Dec 24. Mol Aspects Med. 2015. PMID: 25542748 Free PMC article. Review.
-
Sec61p contributes to signal sequence orientation according to the positive-inside rule.Mol Biol Cell. 2004 Mar;15(3):1470-8. doi: 10.1091/mbc.e03-08-0599. Epub 2003 Dec 10. Mol Biol Cell. 2004. PMID: 14668483 Free PMC article.
-
Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation.J Cell Biol. 2001 May 28;153(5):1061-70. doi: 10.1083/jcb.153.5.1061. J Cell Biol. 2001. PMID: 11381090 Free PMC article.
-
ERAD and protein import defects in a sec61 mutant lacking ER-lumenal loop 7.BMC Cell Biol. 2013 Dec 6;14:56. doi: 10.1186/1471-2121-14-56. BMC Cell Biol. 2013. PMID: 24314051 Free PMC article.
References
-
- Beckmann R, Bubeck D, Grassuci R, Penczek P, Verschoor A, Blobel G, Frank J. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 1997;278:2123–2126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

