GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo
- PMID: 9832509
- PMCID: PMC317242
- DOI: 10.1101/gad.12.22.3579
GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo
Abstract
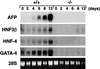
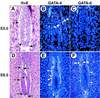
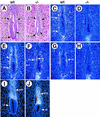
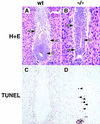
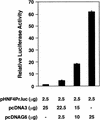
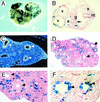
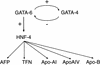
GATA6 belongs to a family of zinc finger transcription factors that play important roles in transducing nuclear events that regulate cellular differentiation and embryonic morphogenesis in vertebrate species. To examine the function of GATA6 during embryonic development, gene targeting was used to generate GATA6-deficient (GATA6(-/-)) ES cells and mice harboring a null mutation in GATA6. Differentiated embryoid bodies derived from GATA6(-/-) ES cells lack a covering layer of visceral endoderm and severely attenuate, or fail to express, genes encoding early and late endodermal markers, including HNF4, GATA4, alpha-fetoprotein (AFP), and HNF3beta. Homozygous GATA6(-/-) mice died between embryonic day (E) 6.5 and E7. 5 and exhibited a specific defect in endoderm differentiation including severely down-regulated expression of GATA4 and absence of HNF4 gene expression. Moreover, widespread programmed cell death was observed within the embryonic ectoderm of GATA6-deficient embryos, a finding also observed in HNF4-deficient embryos. Consistent with these data, forced expression of GATA6 activated the HNF4 promoter in nonendodermal cells. Finally, to examine the function of GATA6 during later embryonic development, GATA6(-/-)-C57BL/6 chimeric mice were generated. lacZ-tagged GATA6(-/-) ES cells contributed to all embryonic tissues with the exception of the endodermally derived bronchial epithelium. Taken together, these data suggest a model in which GATA6 lies upstream of HNF4 in a transcriptional cascade that regulates differentiation of the visceral endoderm. In addition, these data demonstrate that GATA6 is required for establishment of the endodermally derived bronchial epithelium.
Figures
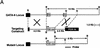
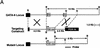
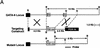
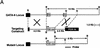


Similar articles
-
The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development.Development. 2001 Feb;128(4):503-11. doi: 10.1242/dev.128.4.503. Development. 2001. PMID: 11171334
-
The gene encoding the mitogen-responsive phosphoprotein Dab2 is differentially regulated by GATA-6 and GATA-4 in the visceral endoderm.J Biol Chem. 2000 Jun 30;275(26):19949-54. doi: 10.1074/jbc.M001331200. J Biol Chem. 2000. PMID: 10779506
-
Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: tetraploid rescue of Hnf-4(-/-) embryos.Development. 1997 Jan;124(2):279-87. doi: 10.1242/dev.124.2.279. Development. 1997. PMID: 9053305
-
HNF4: a central regulator of hepatocyte differentiation and function.Hepatology. 2003 Jun;37(6):1249-53. doi: 10.1053/jhep.2003.50273. Hepatology. 2003. PMID: 12774000 Review. No abstract available.
-
Generation of embryos directly from embryonic stem cells by tetraploid embryo complementation reveals a role for GATA factors in organogenesis.Biochem Soc Trans. 2005 Dec;33(Pt 6):1534-6. doi: 10.1042/BST0331534. Biochem Soc Trans. 2005. PMID: 16246163 Review.
Cited by
-
The notch ligand delta-like 4 regulates multiple stages of early hemato-vascular development.PLoS One. 2012;7(4):e34553. doi: 10.1371/journal.pone.0034553. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514637 Free PMC article.
-
A system to enrich for primitive streak-derivatives, definitive endoderm and mesoderm, from pluripotent cells in culture.PLoS One. 2012;7(6):e38645. doi: 10.1371/journal.pone.0038645. Epub 2012 Jun 11. PLoS One. 2012. PMID: 22701686 Free PMC article.
-
Reciprocal changes in the expression of transcription factors GATA-4 and GATA-6 accompany adrenocortical tumorigenesis in mice and humans.Mol Med. 1999 Jul;5(7):490-501. Mol Med. 1999. PMID: 10449810 Free PMC article.
-
Association of the transcriptional corepressor TIF1beta with heterochromatin protein 1 (HP1): an essential role for progression through differentiation.Genes Dev. 2004 Sep 1;18(17):2147-60. doi: 10.1101/gad.302904. Genes Dev. 2004. PMID: 15342492 Free PMC article.
-
STAT3-Inducible Mouse ESCs: A Model to Study the Role of STAT3 in ESC Maintenance and Lineage Differentiation.Stem Cells Int. 2018 Sep 4;2018:8632950. doi: 10.1155/2018/8632950. eCollection 2018. Stem Cells Int. 2018. PMID: 30254684 Free PMC article.
References
-
- Bradley A. Teratocarcinomas and embryonic stem cells, a practical approach (ed. E.J. Robertson), pp. 113–151. IRL Press, Oxford, UK. 1987. Production and analysis of chimeric mice.
-
- Chang MW, Barr E, Seltzer J, Jiang Y-Q, Nabel GJ, Nabel EG, Parmacek MS, Leiden JM. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 1995;267:518–522. - PubMed
-
- Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes & Dev. 1994;8:2466–2477. - PubMed
-
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
