Role of NK cells in early host response to chlamydial genital infection
- PMID: 9826367
- PMCID: PMC108743
- DOI: 10.1128/IAI.66.12.5867-5875.1998
Role of NK cells in early host response to chlamydial genital infection
Abstract
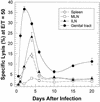
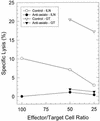
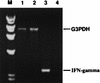
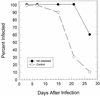
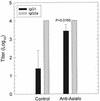
The cell-mediated immune response has been documented to be the major protective immune mechanism in mice infected genitally with the agent of mouse pneumonitis (MoPn), a biovar of Chlamydia trachomatis. Moreover, there is strong evidence to indicate that gamma interferon (IFN-gamma) is a major effector mechanism of the cell-mediated immune response. Previous studies from this laboratory have also reported that the dominant cell population in the genital tract is the CD4 Th1 population. When experiments were performed by the enzyme-linked immunospot assay, high numbers of cells producing IFN-gamma were found in the genital tract, concomitant with resolution of the infection; however, in addition, an increase in IFN-gamma-producing cells which were CD4(-) was seen early in the infection. Since natural killer (NK) cells produce IFN-gamma and have been found to participate in the early responses in other infections, we hypothesized that NK cells are responsible for early IFN-gamma production in the murine chlamydial model. NK cells were quantified by the standard YAC-1 cytotoxicity assay and were found to appear in the genital tract as early as 12 h after intravaginal infection with MoPn. The cells were confirmed to be NK cells by abrogation of YAC-1 cell cytotoxicity by treatment in vitro and in vivo with anti-asialo-GM1. The early IFN-gamma response could also be depleted by treatment with anti-asialo-GM1, indicating that NK cells were responsible for the production of this cytokine. Of interest was our observation that depletion of NK cells also exacerbated the course of infection in the mice and elicited a Th2 response, as indicated by a marked increase in immunoglobulin G1 antibody. Thus, these data demonstrate that NK cells are not only responsible for the production of IFN-gamma early in the course of chlamydial genital tract infection but are also, via IFN-gamma, a significant factor in the development of the Th1 CD4 response and in the control of the infection.
Figures



Similar articles
-
Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis.Infect Immun. 1995 May;63(5):1784-9. doi: 10.1128/iai.63.5.1784-1789.1995. Infect Immun. 1995. PMID: 7729886 Free PMC article.
-
Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis.Infect Immun. 1997 Jul;65(7):2876-82. doi: 10.1128/iai.65.7.2876-2882.1997. Infect Immun. 1997. PMID: 9199462 Free PMC article.
-
Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo.Infect Immun. 2000 Oct;68(10):5587-94. doi: 10.1128/IAI.68.10.5587-5594.2000. Infect Immun. 2000. PMID: 10992458 Free PMC article.
-
Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection.Infect Immun. 1999 Jun;67(6):3019-25. doi: 10.1128/IAI.67.6.3019-3025.1999. Infect Immun. 1999. PMID: 10338514 Free PMC article.
-
Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response.Infect Immun. 1997 Mar;65(3):1032-44. doi: 10.1128/IAI.65.3.1032-1044.1997. Infect Immun. 1997. PMID: 9038313 Free PMC article.
Cited by
-
CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract.J Immunol. 2012 Sep 1;189(5):2441-9. doi: 10.4049/jimmunol.1103032. Epub 2012 Aug 1. J Immunol. 2012. PMID: 22855710 Free PMC article.
-
Murine modeling of menstruation identifies immune correlates of protection during Chlamydia muridarum challenge.bioRxiv [Preprint]. 2024 May 23:2024.05.21.595090. doi: 10.1101/2024.05.21.595090. bioRxiv. 2024. PMID: 38826233 Free PMC article. Preprint.
-
A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant.Vaccines (Basel). 2017 Jan 19;5(1):3. doi: 10.3390/vaccines5010003. Vaccines (Basel). 2017. PMID: 28106821 Free PMC article.
-
Innate IFN-γ Is Essential for Systemic Chlamydia muridarum Control in Mice, While CD4 T Cell-Dependent IFN-γ Production Is Highly Redundant in the Female Reproductive Tract.Infect Immun. 2021 Feb 16;89(3):e00541-20. doi: 10.1128/IAI.00541-20. Print 2021 Feb 16. Infect Immun. 2021. PMID: 33257535 Free PMC article.
-
Modulation of MICA on the surface of Chlamydia trachomatis-infected endocervical epithelial cells promotes NK cell-mediated killing.FEMS Immunol Med Microbiol. 2012 Jun;65(1):32-42. doi: 10.1111/j.1574-695X.2012.00930.x. Epub 2012 Feb 16. FEMS Immunol Med Microbiol. 2012. PMID: 22251247 Free PMC article.
References
-
- Cain, T. K., and R. G. Rank. Unpublished data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

