Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells
- PMID: 9820504
- PMCID: PMC2239007
Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells
Abstract
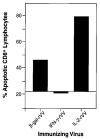
Following an infection or immunization, a primary CD8+ T cell response generally rises then falls rapidly before giving rise to a "memory" response. When we immunized mice with recombinant viral immunogens optimized to enhance the lytic capability of CD8+ T cells, we measured a profound depression in Ag-specific effector function after early restimulation. Indeed, a "mirror image" cytolytic capability was observed: the most powerful immunogens, as measured by cytolytic capacity 6 days after immunization, elicited the weakest secondary immune response when evaluated following an additional 6 days after restimulation. To understand the mechanism of this suppression, we examined the fate of splenocytes immunized with a vaccinia virus encoding Ag and IL-2 then restimulated ex vivo. We found that these splenocytes underwent an apoptotic cell death, upon early restimulation, that was not dependent on the engagement of the FasR (CD95). Unlike previously described mechanisms of "propriocidal cell death" and "clonal exhaustion," the cell death we observed was not an inherent property of the CD8+ T cells but rather was due to a population of splenocytes that stained positive for both the Mac-1 and Gr-1 surface markers. Deletion of these cells in vitro or in vivo completely abrogated the observed suppression of cytolytic reactivity of Ag-specific CD8+ T cells. These observations could account for the apparent absence of Ag-specific immune responses after some current vaccination regimens employing powerful immunogens. Finally, our results may shed new light on a mechanism for the suppression of CD8+ T cell responses and its effect on vaccine efficacy and on immune memory.
Figures






Similar articles
-
TCR/self-antigen interactions drive double-negative T cell peripheral expansion and differentiation into suppressor cells.J Immunol. 2001 Dec 1;167(11):6188-94. doi: 10.4049/jimmunol.167.11.6188. J Immunol. 2001. PMID: 11714779
-
The impact of a boosting immunogen on the differentiation of secondary memory CD8+ T cells.J Virol. 2007 Dec;81(23):12793-802. doi: 10.1128/JVI.01519-07. Epub 2007 Sep 19. J Virol. 2007. PMID: 17881444 Free PMC article.
-
CD4+ CD25+ [corrected] regulatory T cells render naive CD4+ CD25- T cells anergic and suppressive.Immunology. 2007 Apr;120(4):447-55. doi: 10.1111/j.1365-2567.2007.02544.x. Epub 2007 Jan 17. Immunology. 2007. PMID: 17244157 Free PMC article.
-
Emerging concepts in CD8(+) T regulatory cells.Curr Opin Immunol. 2008 Jun;20(3):327-31. doi: 10.1016/j.coi.2008.02.003. Epub 2008 Apr 10. Curr Opin Immunol. 2008. PMID: 18406591 Free PMC article. Review.
-
Exploiting apoptosis for therapeutic tolerance induction.J Immunol. 2013 Dec 1;191(11):5341-6. doi: 10.4049/jimmunol.1302070. J Immunol. 2013. PMID: 24244028 Free PMC article. Review.
Cited by
-
Chinese Medicines and Natural Medicine as Immunotherapeutic Agents for Gastric Cancer: Recent Advances.Cancer Rep (Hoboken). 2024 Sep;7(9):e2134. doi: 10.1002/cnr2.2134. Cancer Rep (Hoboken). 2024. PMID: 39233637 Free PMC article. Review.
-
Cellular constituents of immune escape within the tumor microenvironment.Cancer Res. 2012 Jul 1;72(13):3125-30. doi: 10.1158/0008-5472.CAN-11-4094. Epub 2012 Jun 21. Cancer Res. 2012. PMID: 22721837 Free PMC article. Review.
-
Immune suppression: the hallmark of myeloid derived suppressor cells.Immunol Invest. 2012;41(6-7):581-94. doi: 10.3109/08820139.2012.680635. Immunol Invest. 2012. PMID: 23017136 Free PMC article. Review.
-
Toll-like receptors in tumor immunotherapy.Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5280-9. doi: 10.1158/1078-0432.CCR-07-1378. Clin Cancer Res. 2007. PMID: 17875756 Free PMC article. Review.
-
Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors.Cancer Immunol Immunother. 2008 May;57(5):701-18. doi: 10.1007/s00262-007-0410-4. Epub 2007 Oct 26. Cancer Immunol Immunother. 2008. PMID: 17962945 Free PMC article.
References
-
- Scott DW, Grdina T, Shi Y. T cells commit suicide, but B cells are murdered! J. Immunol. 1996;156:2352. - PubMed
-
- Lenardo MJ. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858. - PubMed
-
- Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139. - PubMed
-
- Welsh RM, Selin LK, Razvi ES. Role of apoptosis in the regulation of virus-induced T cell responses, immune suppression, and memory. J. Cell. Biochem. 1995;59:135. - PubMed
-
- Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
