A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals
- PMID: 9806968
- PMCID: PMC2229444
- DOI: 10.1085/jgp.112.5.593
A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals
Abstract
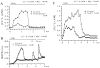
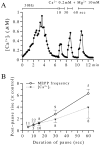
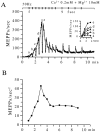
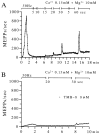
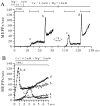
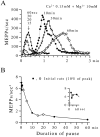
The extent to which Ca2+-induced Ca2+ release (CICR) affects transmitter release is unknown. Continuous nerve stimulation (20-50 Hz) caused slow transient increases in miniature end-plate potential (MEPP) frequency (MEPP-hump) and intracellular free Ca2+ ([Ca2+]i) in presynaptic terminals (Ca2+-hump) in frog skeletal muscles over a period of minutes in a low Ca2+, high Mg2+ solution. Mn2+ quenched Indo-1 and Fura-2 fluorescence, thus indicating that stimulation was accompanied by opening of voltage-dependent Ca2+ channels. MEPP-hump depended on extracellular Ca2+ (0.05-0.2 mM) and stimulation frequency. Both the Ca2+- and MEPP-humps were blocked by 8-(N, N-diethylamino)octyl3,4,5-trimethoxybenzoate hydrochloride (TMB-8), ryanodine, and thapsigargin, but enhanced by CN-. Thus, Ca2+-hump is generated by the activation of CICR via ryanodine receptors by Ca2+ entry, producing MEPP-hump. A short interruption of tetanus (<1 min) during MEPP-hump quickly reduced MEPP frequency to a level attained under the effect of TMB-8 or thapsigargin, while resuming tetanus swiftly raised MEPP frequency to the previous or higher level. Thus, the steady/equilibrium condition balancing CICR and Ca2+ clearance occurs in nerve terminals with slow changes toward a greater activation of CICR (priming) during the rising phase of MEPP-hump and toward a smaller activation during the decay phase. A short pause applied after the end of MEPP- or Ca2+-hump affected little MEPP frequency or [Ca2+]i, but caused a quick increase (faster than MEPP- or Ca2+-hump) after the pause, whose magnitude increased with an increase in pause duration (<1 min), suggesting that Ca2+ entry-dependent inactivation, but not depriming process, explains the decay of the humps. The depriming process was seen by giving a much longer pause (>1 min). Thus, ryanodine receptors in frog motor nerve terminals are endowed with Ca2+ entry-dependent slow priming and fast inactivation mechanisms, as well as Ca2+ entry-dependent activation, and involved in asynchronous exocytosis. Physiological significance of CICR in presynaptic terminals was discussed.
Figures





Similar articles
-
Type-3 ryanodine receptor involved in Ca2+-induced Ca2+ release and transmitter exocytosis at frog motor nerve terminals.Cell Calcium. 2005 Dec;38(6):557-67. doi: 10.1016/j.ceca.2005.07.008. Epub 2005 Sep 12. Cell Calcium. 2005. PMID: 16157373
-
Functional coupling of Ca(2+) channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity.J Gen Physiol. 2000 Apr;115(4):519-32. doi: 10.1085/jgp.115.4.519. J Gen Physiol. 2000. PMID: 10736317 Free PMC article.
-
Calcium dependence of the priming, activation and inactivation of ryanodine receptors in frog motor nerve terminals.Eur J Neurosci. 2010 Sep;32(6):948-62. doi: 10.1111/j.1460-9568.2010.07381.x. Epub 2010 Aug 26. Eur J Neurosci. 2010. PMID: 20796022
-
Coupling of L-type calcium channels to neurotransmitter release at mouse motor nerve terminals.Pflugers Arch. 2001 Mar;441(6):824-31. doi: 10.1007/s004240000489. Pflugers Arch. 2001. PMID: 11316267
-
Membrane excitability and secretion from peptidergic nerve terminals.Cell Mol Neurobiol. 1998 Feb;18(1):45-63. doi: 10.1023/a:1022523109900. Cell Mol Neurobiol. 1998. PMID: 9524729 Review.
Cited by
-
The Putative Drosophila TMEM184B Ortholog Tmep Ensures Proper Locomotion by Restraining Ectopic Firing at the Neuromuscular Junction.Mol Neurobiol. 2022 Apr;59(4):2605-2619. doi: 10.1007/s12035-022-02760-3. Epub 2022 Feb 2. Mol Neurobiol. 2022. PMID: 35107803 Free PMC article.
-
cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells.J Physiol. 2001 Oct 15;536(Pt 2):375-85. doi: 10.1111/j.1469-7793.2001.0375c.xd. J Physiol. 2001. PMID: 11600673 Free PMC article.
-
A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14536-41. doi: 10.1073/pnas.212511399. Epub 2002 Oct 18. Proc Natl Acad Sci U S A. 2002. PMID: 12388783 Free PMC article.
-
Differential regulation of transmitter release by presynaptic and glial Ca2+ internal stores at the neuromuscular synapse.J Neurosci. 2001 Mar 15;21(6):1911-22. doi: 10.1523/JNEUROSCI.21-06-01911.2001. J Neurosci. 2001. PMID: 11245676 Free PMC article.
-
Ca2+ syntillas, miniature Ca2+ release events in terminals of hypothalamic neurons, are increased in frequency by depolarization in the absence of Ca2+ influx.J Neurosci. 2004 Feb 4;24(5):1226-35. doi: 10.1523/JNEUROSCI.4286-03.2004. J Neurosci. 2004. PMID: 14762141 Free PMC article.
References
-
- Bernardi P, Veronese P, Petronilli V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. J Biol Chem. 1993;268:1005–1010. - PubMed
-
- Berridge MJ. Inositoltrisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium response curves of Ins (1,4,5-) P3 - and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

