Localization of myosin-Ibeta near both ends of tip links in frog saccular hair cells
- PMID: 9786971
- PMCID: PMC6793541
- DOI: 10.1523/JNEUROSCI.18-21-08637.1998
Localization of myosin-Ibeta near both ends of tip links in frog saccular hair cells
Abstract
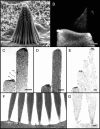
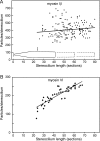
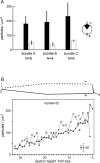
Current evidence suggests that the adaptation motor of mechanoelectrical transduction in vertebrate hair cells is myosin-Ibeta. Previously, confocal and electron microscopy of bullfrog saccular hair cells using an anti-myosin-Ibeta antibody labeled the tips of stereocilia. We have now done quantitative immunoelectron microscopy to test whether myosin-Ibeta is enriched at or near the side plaques of tip links, the proposed sites of adaptation, using hair bundles that were serially sectioned parallel to the macular surface. The highest particle density occurred at stereocilia bases, close to the cuticular plate. Also, stereocilia of differing lengths had approximately the same number of total particles, suggesting equal targeting of myosin-Ibeta to all stereocilia. Finally, particles tended to clump in clusters of two to five particles in the distal two-thirds of stereocilia, suggesting a tendency for self-assembly of myosin-Ibeta. As expected from fluorescence microscopy, particle density was high in the distal 1 micrometer of stereocilia. If myosin-Ibeta is the adaptation motor, a difference should exist in particle density between regions containing the side plaque and those excluding it. Averaging of particle distributions revealed two regions with approximately twice the average density: at the upper ends of tip links in a 700-nm-long region centered approximately 100 nm above the side plaque, and at the lower ends of tip links within the tip plaques. Controls demonstrated no such increase. The shortest stereocilia, which lack side plaques, showed no concentration rise on their sides. Thus, the specific localization of myosin-Ibeta at both ends of tip links supports its role as the adaptation motor.
Figures





Similar articles
-
Myosin Ibeta is located at tip link anchors in vestibular hair bundles.J Neurosci. 1998 Jun 15;18(12):4603-15. doi: 10.1523/JNEUROSCI.18-12-04603.1998. J Neurosci. 1998. PMID: 9614235 Free PMC article.
-
Immunolocalization of myosin Ibeta in the hair cell's hair bundle.Cell Motil Cytoskeleton. 1998;39(2):159-65. doi: 10.1002/(SICI)1097-0169(1998)39:2<159::AID-CM6>3.0.CO;2-1. Cell Motil Cytoskeleton. 1998. PMID: 9484957
-
Unconventional myosins in inner-ear sensory epithelia.J Cell Biol. 1997 Jun 16;137(6):1287-307. doi: 10.1083/jcb.137.6.1287. J Cell Biol. 1997. PMID: 9182663 Free PMC article.
-
Mechanoelectrical transduction by hair cells of the bullfrog's sacculus.Prog Brain Res. 1989;80:129-35; discussion 127-8. doi: 10.1016/s0079-6123(08)62206-2. Prog Brain Res. 1989. PMID: 2699361 Review.
-
When size matters: the dynamic regulation of stereocilia lengths.Curr Opin Cell Biol. 2005 Feb;17(1):55-61. doi: 10.1016/j.ceb.2004.12.005. Curr Opin Cell Biol. 2005. PMID: 15661519 Review.
Cited by
-
Regulation of the transient receptor potential channel TRPA1 by its N-terminal ankyrin repeat domain.J Mol Model. 2013 Nov;19(11):4689-700. doi: 10.1007/s00894-012-1505-1. Epub 2012 Jul 3. J Mol Model. 2013. PMID: 22752543
-
A cryo-tomography-based volumetric model of the actin core of mouse vestibular hair cell stereocilia lacking plastin 1.J Struct Biol. 2020 Apr 1;210(1):107461. doi: 10.1016/j.jsb.2020.107461. Epub 2020 Jan 18. J Struct Biol. 2020. PMID: 31962158 Free PMC article.
-
Tip links in hair cells: molecular composition and role in hearing loss.Curr Opin Otolaryngol Head Neck Surg. 2009 Oct;17(5):388-93. doi: 10.1097/MOO.0b013e3283303472. Curr Opin Otolaryngol Head Neck Surg. 2009. PMID: 19633555 Free PMC article. Review.
-
Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells.Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):11765-72. doi: 10.1073/pnas.97.22.11765. Proc Natl Acad Sci U S A. 2000. PMID: 11050207 Free PMC article.
-
Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells.J Neurosci. 2007 Dec 12;27(50):13890-902. doi: 10.1523/JNEUROSCI.2159-07.2007. J Neurosci. 2007. PMID: 18077701 Free PMC article.
References
-
- Assad JA, Shepherd GMG, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. - PubMed
-
- Denk W, Holt JR, Shepherd GMG, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
