Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice
- PMID: 9765468
- PMCID: PMC110340
- DOI: 10.1128/JVI.72.11.9208-9216.1998
Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice
Abstract
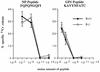
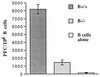
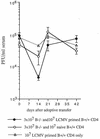
Adoptive transfer of virus-specific memory lymphocytes can be used to identify factors and mechanisms involved in the clearance of persistent virus infections. To analyze the role of B cells in clearing persistent infection with lymphocytic choriomeningitis virus (LCMV), we used B-cell-deficient muMT/muMT (B-/-) mice. B-/- mice controlled an acute LCMV infection with the same kinetics and efficiency as B-cell-competent (B+/+) mice via virus-specific, major histocompatibility complex (MHC) class I-restricted CD8(+) cytotoxic T lymphocytes (CTL). CTL from B-/- and B+/+ mice were equivalent in affinity to known LCMV CTL epitopes and had similar CTL precursor frequencies (pCTL). Adoptive transfer of memory cells from B+/+ mice led to virus clearance from persistently infected B+/+ recipients even after in vitro depletion of B cells, indicating that B cells or immunoglobulins are not required in the transfer population. In contrast, transfer of memory splenocytes from B-/- mice failed to clear virus. Control of virus was restored neither by transferring higher numbers of pCTL nor by supplementing B-/- memory splenocytes with LCMV-immune B cells or immune sera. Instead, B-/- mice were found to have a profound CD4 helper defect. Furthermore, compared to cultured splenocytes from B+/+ mice, those from B-/- mice secreted less gamma interferon (IFN-gamma) and interleukin 2, with differences most pronounced for CD8 T cells. While emphasizing the importance of CD4 T-cell help and IFN-gamma in the control of persistent infections, the CD4 T-helper and CD8 T-cell defects in B-/- mice suggest that B cells contribute to the induction of competent T effector cells.
Figures
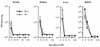


Similar articles
-
A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers.Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6874-9. doi: 10.1073/pnas.94.13.6874. Proc Natl Acad Sci U S A. 1997. PMID: 9192659 Free PMC article.
-
CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection.J Virol. 1994 Dec;68(12):8056-63. doi: 10.1128/JVI.68.12.8056-8063.1994. J Virol. 1994. PMID: 7966595 Free PMC article.
-
CD8 T cell memory in B cell-deficient mice.J Exp Med. 1996 May 1;183(5):2165-74. doi: 10.1084/jem.183.5.2165. J Exp Med. 1996. PMID: 8642326 Free PMC article.
-
Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review.
-
Lymphocytic choriomeningitis infection of the central nervous system.Front Biosci. 2008 May 1;13:4529-43. doi: 10.2741/3021. Front Biosci. 2008. PMID: 18508527 Free PMC article. Review.
Cited by
-
Expression and regulation of chemokines in murine and human type 1 diabetes.Diabetes. 2012 Feb;61(2):436-46. doi: 10.2337/db11-0853. Epub 2011 Dec 30. Diabetes. 2012. PMID: 22210319 Free PMC article.
-
Class switching and high-affinity immunoglobulin G production by B cells is dispensable for the development of hypertension in mice.Cardiovasc Res. 2021 Mar 21;117(4):1217-1228. doi: 10.1093/cvr/cvaa187. Cardiovasc Res. 2021. PMID: 32609312 Free PMC article.
-
Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain.Infect Immun. 1999 Nov;67(11):6002-7. doi: 10.1128/IAI.67.11.6002-6007.1999. Infect Immun. 1999. PMID: 10531260 Free PMC article.
-
Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms.J Virol. 2010 Sep;84(18):9217-26. doi: 10.1128/JVI.01069-10. Epub 2010 Jun 30. J Virol. 2010. PMID: 20592069 Free PMC article.
-
Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells.Eur J Immunol. 2008 Dec;38(12):3411-25. doi: 10.1002/eji.200838432. Eur J Immunol. 2008. PMID: 19009526 Free PMC article.
References
-
- Amigorena S, Bonnerot C. Role of B-cell and Fc receptors in the selection of T-cell epitopes. Curr Opin Immunol. 1998;10:88–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

