The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2
- PMID: 9765401
- PMCID: PMC110273
- DOI: 10.1128/JVI.72.11.8620-8626.1998
The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2
Abstract
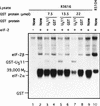
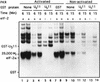
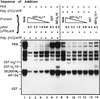
In herpes simplex virus-infected cells, viral gamma134.5 protein blocks the shutoff of protein synthesis by activated protein kinase R (PKR) by directing the protein phosphatase 1alpha to dephosphorylate the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2alpha). The amino acid sequence of the gamma134.5 protein which interacts with the phosphatase has high homology to a domain of the eukaryotic protein GADD34. A class of compensatory mutants characterized by a deletion which results in the juxtaposition of the alpha47 promoter next to US11, a gamma2 (late) gene in wild-type virus-infected cells, has been described. In cells infected with these mutants, protein synthesis continues even in the absence of the gamma134.5 gene. In these cells, PKR is activated but eIF-2alpha is not phosphorylated, and the phosphatase is not redirected to dephosphorylate eIF-2alpha. We report the following: (i) in cells infected with these mutants, US11 protein was made early in infection; (ii) US11 protein bound PKR and was phosphorylated; (iii) in in vitro assays, US11 blocked the phosphorylation of eIF-2alpha by PKR activated by poly(I-C); and (iv) US11 was more effective if present in the reaction mixture during the activation of PKR than if added after PKR had been activated by poly(I-C). We conclude the following: (i) in cells infected with the compensatory mutants, US11 made early in infection binds to PKR and precludes the phosphorylation of eIF-2alpha, whereas US11 driven by its natural promoter and expressed late in infection is ineffective; and (ii) activation of PKR by double-stranded RNA is a common impediment countered by most viruses by different mechanisms. The gamma134.5 gene is not highly conserved among herpesviruses. A likely scenario is that acquisition by a progenitor of herpes simplex virus of a portion of the cellular GADD34 gene resulted in a more potent and reliable means of curbing the effects of activated PKR. US11 was retained as a gamma2 gene because, like many viral proteins, it has multiple functions.
Figures


Similar articles
-
The second-site mutation in the herpes simplex virus recombinants lacking the gamma134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2alpha.J Virol. 1998 Sep;72(9):7005-11. doi: 10.1128/JVI.72.9.7005-7011.1998. J Virol. 1998. PMID: 9696792 Free PMC article.
-
The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase.Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):843-8. doi: 10.1073/pnas.94.3.843. Proc Natl Acad Sci U S A. 1997. PMID: 9023344 Free PMC article.
-
Second-site mutation outside of the U(S)10-12 domain of Deltagamma(1)34.5 herpes simplex virus 1 recombinant blocks the shutoff of protein synthesis induced by activated protein kinase R and partially restores neurovirulence.J Virol. 2002 Feb;76(3):942-9. doi: 10.1128/jvi.76.3.942-949.2002. J Virol. 2002. PMID: 11773369 Free PMC article.
-
Neutralizing innate host defenses to control viral translation in HSV-1 infected cells.Int Rev Immunol. 2004 Jan-Apr;23(1-2):199-220. doi: 10.1080/08830180490265600. Int Rev Immunol. 2004. PMID: 14690861 Review.
-
Viral proteins targeting host protein kinase R to evade an innate immune response: a mini review.Biotechnol Genet Eng Rev. 2018 Apr;34(1):33-59. doi: 10.1080/02648725.2018.1467151. Epub 2018 May 2. Biotechnol Genet Eng Rev. 2018. PMID: 29716441 Review.
Cited by
-
Strategies for engineering oncolytic viruses to enhance cancer immunotherapy.Front Pharmacol. 2024 Sep 6;15:1450203. doi: 10.3389/fphar.2024.1450203. eCollection 2024. Front Pharmacol. 2024. PMID: 39309012 Free PMC article. Review.
-
Suppression of the interferon-mediated innate immune response by pseudorabies virus.J Virol. 2006 Jul;80(13):6345-56. doi: 10.1128/JVI.00554-06. J Virol. 2006. PMID: 16775323 Free PMC article.
-
Human BAG-1 proteins bind to the cellular stress response protein GADD34 and interfere with GADD34 functions.Mol Cell Biol. 2003 May;23(10):3477-86. doi: 10.1128/MCB.23.10.3477-3486.2003. Mol Cell Biol. 2003. PMID: 12724406 Free PMC article.
-
Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey.J Virol. 2003 Jun;77(11):6167-77. doi: 10.1128/jvi.77.11.6167-6177.2003. J Virol. 2003. PMID: 12743273 Free PMC article.
-
Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors.J Virol. 2006 Aug;80(15):7308-15. doi: 10.1128/JVI.00725-06. J Virol. 2006. PMID: 16840311 Free PMC article.
References
-
- Brand S R, Kobayashi R, Mathews M B. The tat protein in human immunodeficiency virus type 1 is a substrate and inhibitor of the inhibitor of the interferon-induced, virally activated protein kinase, PKR. J Biol Chem. 1997;272:8388–8395. - PubMed
-
- Carroll K, Elroy-Stein O, Moss B, Jagus R. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2α-specific protein kinase. J Biol Chem. 1993;268:12837–12842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

