Delayed release of neurotransmitter from cerebellar granule cells
- PMID: 9763467
- PMCID: PMC6792848
- DOI: 10.1523/JNEUROSCI.18-20-08214.1998
Delayed release of neurotransmitter from cerebellar granule cells
Abstract
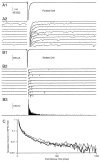
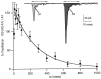
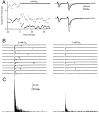
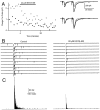
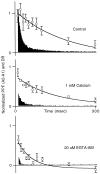
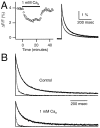
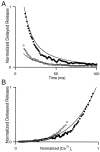
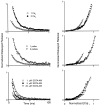
At fast chemical synapses the rapid release of neurotransmitter that occurs within a few milliseconds of an action potential is followed by a more sustained elevation of release probability, known as delayed release. Here we characterize the role of calcium in delayed release and test the hypothesis that facilitation and delayed release share a common mechanism. Synapses between cerebellar granule cells and their postsynaptic targets, stellate cells and Purkinje cells, were studied in rat brain slices. Presynaptic calcium transients were measured with calcium-sensitive fluorophores, and delayed release was detected with whole-cell recordings. Calcium influx, presynaptic calcium dynamics, and the number of stimulus pulses were altered to assess their effect on delayed release and facilitation. Following single stimuli, delayed release can be separated into two components: one lasting for tens of milliseconds that is steeply calcium-dependent, the other lasting for hundreds of milliseconds that is driven by low levels of calcium with a nearly linear calcium dependence. The amplitude, calcium dependence, and magnitude of delayed release do not correspond to those of facilitation, indicating that these processes are not simple reflections of a shared mechanism. The steep calcium dependence of delayed release, combined with the large calcium transients observed in these presynaptic terminals, suggests that for physiological conditions delayed release provides a way for cells to influence their postsynaptic targets long after their own action potential activity has subsided.
Figures



Similar articles
-
Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses.J Neurosci. 2002 Jan 1;22(1):21-8. doi: 10.1523/JNEUROSCI.22-01-00021.2002. J Neurosci. 2002. PMID: 11756484 Free PMC article.
-
Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse.J Neurosci. 1997 May 15;17(10):3425-35. doi: 10.1523/JNEUROSCI.17-10-03425.1997. J Neurosci. 1997. PMID: 9133368 Free PMC article.
-
Presynaptic strontium dynamics and synaptic transmission.Biophys J. 1999 Apr;76(4):2029-42. doi: 10.1016/S0006-3495(99)77360-1. Biophys J. 1999. PMID: 10096899 Free PMC article.
-
Contributions of residual calcium to fast synaptic transmission.J Neurosci. 1999 Aug 1;19(15):6257-66. doi: 10.1523/JNEUROSCI.19-15-06257.1999. J Neurosci. 1999. PMID: 10414955 Free PMC article.
-
The Ever-Growing Puzzle of Asynchronous Release.Front Cell Neurosci. 2019 Feb 12;13:28. doi: 10.3389/fncel.2019.00028. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30809127 Free PMC article. Review.
Cited by
-
Bayesian analysis of the kinetics of quantal transmitter secretion at the neuromuscular junction.J Comput Neurosci. 2015 Oct;39(2):119-29. doi: 10.1007/s10827-015-0567-3. Epub 2015 Jul 2. J Comput Neurosci. 2015. PMID: 26129670
-
Integration of asynchronously released quanta prolongs the postsynaptic spike window.J Neurosci. 2007 Jun 20;27(25):6684-91. doi: 10.1523/JNEUROSCI.0934-07.2007. J Neurosci. 2007. PMID: 17581955 Free PMC article.
-
Frequency-dependent modulation of retinogeniculate transmission by serotonin.J Neurosci. 2004 Dec 1;24(48):10950-62. doi: 10.1523/JNEUROSCI.3749-04.2004. J Neurosci. 2004. PMID: 15574745 Free PMC article.
-
Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities.EMBO J. 2002 Feb 1;21(3):270-80. doi: 10.1093/emboj/21.3.270. EMBO J. 2002. PMID: 11823420 Free PMC article.
-
Upregulated Ca2+ Release from the Endoplasmic Reticulum Leads to Impaired Presynaptic Function in Familial Alzheimer's Disease.Cells. 2022 Jul 11;11(14):2167. doi: 10.3390/cells11142167. Cells. 2022. PMID: 35883609 Free PMC article.
References
-
- Atluri PP, Regehr WG. Differential calcium dependence of facilitation and asynchronous release at a cerebellar synapse. Soc Neurosci Abstr. 1997;23:467.11.
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
-
- Bertram R, Sherman A, Stanley EF. Single-domain/bound calcium hypothesis of transmitter release and facilitation. J Neurophysiol. 1996;75:1919–1931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
