The structural protein ODV-EC27 of Autographa californica nucleopolyhedrovirus is a multifunctional viral cyclin
- PMID: 9736714
- PMCID: PMC21620
- DOI: 10.1073/pnas.95.19.11205
The structural protein ODV-EC27 of Autographa californica nucleopolyhedrovirus is a multifunctional viral cyclin
Abstract
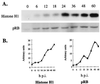
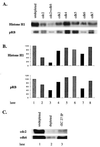
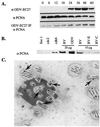
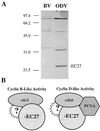
Two major characteristics of baculovirus infection are arrest of the host cell at G2/M phase of the cell cycle with continuing viral DNA replication. We show that Autographa californica nucleopolyhedrovirus (AcMNPV) encodes for a multifunctional cyclin that may partially explain the molecular basis of these important characteristics of AcMNPV (baculovirus) infection. Amino acids 80-110 of the viral structural protein ODV-EC27 (-EC27) demonstrate 25-30% similarity with cellular cyclins within the cyclin box. Immunoprecipitation results using antibodies to -EC27 show that -EC27 can associate with either cdc2 or cdk6 resulting in active kinase complexes that can phosphorylate histone H1 and retinoblastoma protein in vitro. The cdk6-EC27 complex also associates with proliferating cell nuclear antigen (PCNA) and we demonstrate that PCNA is a structural protein of both the budded virus and the occlusion-derived virus. These results suggest that -EC27 can function as a multifunctional cyclin: when associated with cdc2, it exhibits cyclin B-like activity; when associated with cdk6, the complex possesses cyclin D-like activity and binds PCNA. The possible roles of such a multifunctional cyclin during the life cycle of baculovirus are discussed, along with potential implications relative to the expression of functionally authentic recombinant proteins by using baculovirus-infected cells.
Figures

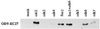
Similar articles
-
Autographa californica nucleopolyhedrovirus infection results in Sf9 cell cycle arrest at G2/M phase.Virology. 1998 Apr 25;244(1):195-211. doi: 10.1006/viro.1998.9097. Virology. 1998. PMID: 9581791
-
Identification of BV/ODV-C42, an Autographa californica nucleopolyhedrovirus orf101-encoded structural protein detected in infected-cell complexes with ODV-EC27 and p78/83.J Virol. 2001 Dec;75(24):12331-8. doi: 10.1128/JVI.75.24.12331-12338.2001. J Virol. 2001. PMID: 11711623 Free PMC article.
-
Transcription, translation, and cellular localization of three Autographa californica nuclear polyhedrosis virus structural proteins: ODV-E18, ODV-E35, and ODV-EC27.Virology. 1996 Aug 1;222(1):100-14. doi: 10.1006/viro.1996.0401. Virology. 1996. PMID: 8806491
-
The cell cycle kinases.Semin Cancer Biol. 1994 Aug;5(4):305-13. Semin Cancer Biol. 1994. PMID: 7803767 Review.
-
Molecular biology of the baculovirus occlusion-derived virus envelope.Curr Drug Targets. 2007 Oct;8(10):1084-95. doi: 10.2174/138945007782151315. Curr Drug Targets. 2007. PMID: 17979668 Review.
Cited by
-
Virologs, viral mimicry, and virocell metabolism: the expanding scale of cellular functions encoded in the complex genomes of giant viruses.FEMS Microbiol Rev. 2023 Sep 5;47(5):fuad053. doi: 10.1093/femsre/fuad053. FEMS Microbiol Rev. 2023. PMID: 37740576 Free PMC article.
-
Orchestrated efforts on host network hijacking: Processes governing virus replication.Virulence. 2020 Dec;11(1):183-198. doi: 10.1080/21505594.2020.1726594. Virulence. 2020. PMID: 32050846 Free PMC article. Review.
-
Baculovirus LEF-11 Hijack Host ATPase ATAD3A to Promote Virus Multiplication in Bombyx mori cells.Sci Rep. 2017 Apr 10;7:46187. doi: 10.1038/srep46187. Sci Rep. 2017. PMID: 28393927 Free PMC article.
-
The conserved cysteines at position 18, 36, and 49 of Autographa californica multiple nucleopolyhedrovirus VP39 are essential for virus replication.Virus Genes. 2024 Dec;60(6):711-724. doi: 10.1007/s11262-024-02111-5. Epub 2024 Oct 6. Virus Genes. 2024. PMID: 39369371
-
Toward system-level understanding of baculovirus-host cell interactions: from molecular fundamental studies to large-scale proteomics approaches.Front Microbiol. 2012 Nov 9;3:391. doi: 10.3389/fmicb.2012.00391. eCollection 2012. Front Microbiol. 2012. PMID: 23162544 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

