Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys
- PMID: 9733899
- PMCID: PMC110239
- DOI: 10.1128/JVI.72.10.8437-8445.1998
Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys
Abstract
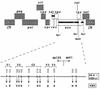
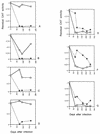
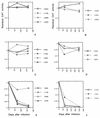
We characterized human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein epitopes recognized by neutralizing antibodies from monkeys recently infected by molecularly cloned simian-human immunodeficiency virus (SHIV) variants. The early neutralizing antibody response in each infected animal was directed mainly against a single epitope. This primary neutralizing epitope, however, differed among individual monkeys infected by identical viruses. Two such neutralization epitopes were determined by sequences in the V2 and V3 loops of the gp120 envelope glycoprotein, while a third neutralization epitope, apparently discontinuous, was determined by both V2 and V3 sequences. These results indicate that the early neutralizing antibody response in SHIV-infected monkeys is monospecific and directed against epitopes composed of the gp120 V2 and V3 variable loops.
Figures
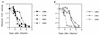


Similar articles
-
Importance of the V1/V2 loop region of simian-human immunodeficiency virus envelope glycoprotein gp120 in determining the strain specificity of the neutralizing antibody response.J Virol. 2008 Nov;82(22):11054-65. doi: 10.1128/JVI.01341-08. Epub 2008 Sep 3. J Virol. 2008. PMID: 18768967 Free PMC article.
-
Induction of antibodies in guinea pigs and rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains.J Virol. 2000 Jan;74(1):254-63. doi: 10.1128/jvi.74.1.254-263.2000. J Virol. 2000. PMID: 10590113 Free PMC article.
-
Development of Broadly Neutralizing Antibodies and Their Mapping by Monomeric gp120 in Human Immunodeficiency Virus Type 1-Infected Humans and Simian-Human Immunodeficiency Virus SHIVSF162P3N-Infected Macaques.J Virol. 2016 Mar 28;90(8):4017-4031. doi: 10.1128/JVI.02898-15. Print 2016 Apr. J Virol. 2016. PMID: 26842476 Free PMC article.
-
Neutralising epitopes of simian immunodeficiency virus envelope glycoprotein.J Med Primatol. 1995 May;24(3):145-9. doi: 10.1111/j.1600-0684.1995.tb00160.x. J Med Primatol. 1995. PMID: 8751054 Review.
-
GP120: target for neutralizing HIV-1 antibodies.Annu Rev Immunol. 2006;24:739-69. doi: 10.1146/annurev.immunol.24.021605.090557. Annu Rev Immunol. 2006. PMID: 16551265 Review.
Cited by
-
Determinants of neutralization resistance in the envelope glycoproteins of a simian-human immunodeficiency virus passaged in vivo.J Virol. 1999 Oct;73(10):8873-9. doi: 10.1128/JVI.73.10.8873-8879.1999. J Virol. 1999. PMID: 10482646 Free PMC article.
-
Quaternary epitope specificities of anti-HIV-1 neutralizing antibodies generated in rhesus macaques infected by the simian/human immunodeficiency virus SHIVSF162P4.J Virol. 2010 Apr;84(7):3443-53. doi: 10.1128/JVI.02617-09. Epub 2010 Jan 27. J Virol. 2010. PMID: 20106929 Free PMC article.
-
Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo.J Virol. 2000 May;74(9):4433-40. doi: 10.1128/jvi.74.9.4433-4440.2000. J Virol. 2000. PMID: 10756060 Free PMC article.
-
Consistent patterns of change during the divergence of human immunodeficiency virus type 1 envelope from that of the inoculated virus in simian/human immunodeficiency virus-infected macaques.J Virol. 2006 Jan;80(2):999-1014. doi: 10.1128/JVI.80.2.999-1014.2006. J Virol. 2006. PMID: 16379001 Free PMC article.
-
Variability in the human immunodeficiency virus type 1 gp120 Env protein linked to phenotype-associated changes in the V3 loop.J Virol. 2002 Apr;76(8):3852-64. doi: 10.1128/jvi.76.8.3852-3864.2002. J Virol. 2002. PMID: 11907225 Free PMC article.
References
-
- Alizon M, Wain-Hobson S, Montagnier L, Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986;46:63–74. - PubMed
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Andeweg A C, Boers P H, Osterhaus A D, Bosch M L. Impact of natural sequence variation in the V2 region of the envelope protein of human immunodeficiency virus type 1 on syncytium induction: a mutational analysis. J Gen Virol. 1995;76:1901–1907. - PubMed
-
- Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

