Transactivation by the E2 protein of oncogenic human papillomavirus type 31 is not essential for early and late viral functions
- PMID: 9733852
- PMCID: PMC110149
- DOI: 10.1128/JVI.72.10.8115-8123.1998
Transactivation by the E2 protein of oncogenic human papillomavirus type 31 is not essential for early and late viral functions
Abstract
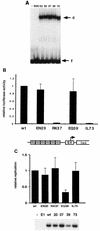
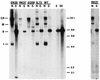
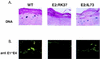
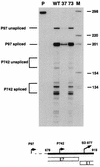
The activation of transcription and of DNA replication are, in some cases, mediated by the same proteins. A prime example is the E2 protein of human papillomaviruses (HPVs), which binds ACCN6GGT sequences and activates heterologous promoters from multimerized binding sites. The E2 protein also has functions in replication, where it complexes with the virally encoded origin recognition protein, E1. Much of the information on these activities is based on transient-transfection assays as well as biochemical analyses; however, their importance in the productive life cycle of oncogenic HPVs remains unclear. To determine the contributions of these E2 functions to the HPV life cycle, a genetic analysis was performed by using an organotypic tissue culture model. HPV type 31 (HPV31) genomes that contained mutations in the N terminus of E2 (amino acid 73) were constructed; these mutants retained replication activities but were transactivation defective. Following transfection of normal human keratinocytes, these mutant genomes were established as stable episomes and expressed early viral transcripts at levels similar to those of wild-type HPV31. Upon differentiation in organotypic raft cultures, the induction of late gene expression and amplification of viral DNA were detected in cell lines harboring mutant genomes. Interestingly, only a modest reduction in late gene expression was observed in the mutant lines. We conclude that the transactivation function of E2 is not essential for the viral life cycle of oncogenic HPVs, although it may act to moderately augment late expression. Our studies suggest that the primary positive role of E2 in the viral life cycle is as a replication factor.
Figures


Similar articles
-
The E8E2C protein, a negative regulator of viral transcription and replication, is required for extrachromosomal maintenance of human papillomavirus type 31 in keratinocytes.J Virol. 2000 Feb;74(3):1178-86. doi: 10.1128/jvi.74.3.1178-1186.2000. J Virol. 2000. PMID: 10627528 Free PMC article.
-
Human papillomavirus type 31 replication modes during the early phases of the viral life cycle depend on transcriptional and posttranscriptional regulation of E1 and E2 expression.J Virol. 2002 Mar;76(5):2263-73. doi: 10.1128/jvi.76.5.2263-2273.2002. J Virol. 2002. PMID: 11836404 Free PMC article.
-
Differential requirements for conserved E2 binding sites in the life cycle of oncogenic human papillomavirus type 31.J Virol. 1998 Feb;72(2):1071-7. doi: 10.1128/JVI.72.2.1071-1077.1998. J Virol. 1998. PMID: 9445001 Free PMC article.
-
Keratinocyte Differentiation-Dependent Human Papillomavirus Gene Regulation.Viruses. 2017 Aug 30;9(9):245. doi: 10.3390/v9090245. Viruses. 2017. PMID: 28867768 Free PMC article. Review.
-
Control of viral replication and transcription by the papillomavirus E8^E2 protein.Virus Res. 2017 Mar 2;231:96-102. doi: 10.1016/j.virusres.2016.11.005. Epub 2016 Nov 4. Virus Res. 2017. PMID: 27825778 Review.
Cited by
-
The E8E2C protein, a negative regulator of viral transcription and replication, is required for extrachromosomal maintenance of human papillomavirus type 31 in keratinocytes.J Virol. 2000 Feb;74(3):1178-86. doi: 10.1128/jvi.74.3.1178-1186.2000. J Virol. 2000. PMID: 10627528 Free PMC article.
-
Use of Cap Analysis Gene Expression to detect human papillomavirus promoter activity patterns at different disease stages.Sci Rep. 2020 Oct 22;10(1):17991. doi: 10.1038/s41598-020-75133-2. Sci Rep. 2020. PMID: 33093512 Free PMC article.
-
Differentiation-dependent chromatin rearrangement coincides with activation of human papillomavirus type 31 late gene expression.J Virol. 2001 Oct;75(20):10005-13. doi: 10.1128/JVI.75.20.10005-10013.2001. J Virol. 2001. PMID: 11559836 Free PMC article.
-
Bromodomain protein 4 mediates the papillomavirus E2 transcriptional activation function.J Virol. 2006 May;80(9):4276-85. doi: 10.1128/JVI.80.9.4276-4285.2006. J Virol. 2006. PMID: 16611886 Free PMC article.
-
Expression of the HPV11 E2 gene in transgenic mice does not result in alterations of the phenotypic pattern.Transgenic Res. 2008 Feb;17(1):1-8. doi: 10.1007/s11248-007-9130-y. Epub 2007 Aug 16. Transgenic Res. 2008. PMID: 17701441
References
-
- Alderborn A, Jareborg N, Burnett S. Evidence that the transcriptional trans-activating function of the bovine papillomavirus type 1 E2 gene is not required for viral DNA amplification in division-arrested cells. J Gen Virol. 1992;73:2639–2651. - PubMed
-
- Androphy E J, Lowy D R, Schiller J T. Bovine papillomavirus E2 trans-activating gene product binds to specific sites in papillomavirus DNA. Nature. 1987;325:70–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

