Prenylcysteine alpha-carboxyl methyltransferase in suspension-cultured tobacco cells
- PMID: 9733531
- PMCID: PMC34848
- DOI: 10.1104/pp.118.1.115
Prenylcysteine alpha-carboxyl methyltransferase in suspension-cultured tobacco cells
Abstract
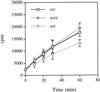
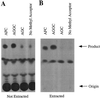
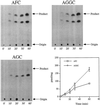
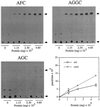
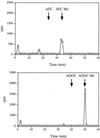
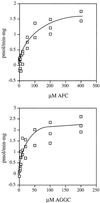
Isoprenylation is a posttranslational modification that is believed to be necessary, but not sufficient, for the efficient association of numerous eukaryotic cell proteins with membranes. Additional modifications have been shown to be required for proper intracellular targeting and function of certain isoprenylated proteins in mammalian and yeast cells. Although protein isoprenylation has been demonstrated in plants, postisoprenylation processing of plant proteins has not been described. Here we demonstrate that cultured tobacco (Nicotiana tabacum cv Bright Yellow-2) cells contain farnesylcysteine and geranylgeranylcysteine alpha-carboxyl methyltransferase activities with apparent Michaelis constants of 73 and 21 &mgr;M for N-acetyl-S-trans, trans-farnesyl-L-cysteine and N-acetyl-S-all-trans-geranylgeranyl-L-cysteine, respectively. Furthermore, competition analysis indicates that the same enzyme is responsible for both activities. These results suggest that alpha-carboxyl methylation is a step in the maturation of isoprenylated proteins in plants.
Figures

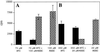
Similar articles
-
Identification and functional expression in yeast of a prenylcysteine alpha-carboxyl methyltransferase gene from Arabidopsis thaliana.Plant Mol Biol. 2001 Mar;45(4):469-76. doi: 10.1023/a:1010671202925. Plant Mol Biol. 2001. PMID: 11352465
-
A single activity carboxyl methylates both farnesyl and geranylgeranyl cysteine residues.FEBS Lett. 1991 Dec 16;295(1-3):189-94. doi: 10.1016/0014-5793(91)81415-5. FEBS Lett. 1991. PMID: 1765152
-
10 Genetic approaches to understanding the physiologic importance of the carboxyl methylation of isoprenylated proteins.Enzymes. 2006;24:273-301. doi: 10.1016/S1874-6047(06)80012-0. Epub 2007 Jun 4. Enzymes. 2006. PMID: 26718044
-
Protein isoprenylation: the fat of the matter.Trends Plant Sci. 2009 Mar;14(3):163-70. doi: 10.1016/j.tplants.2008.12.001. Epub 2009 Feb 7. Trends Plant Sci. 2009. PMID: 19201644 Review.
-
Posttranslational isoprenylation of tryptophan in bacteria.Beilstein J Org Chem. 2017 Feb 22;13:338-346. doi: 10.3762/bjoc.13.37. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28326143 Free PMC article. Review.
Cited by
-
Identification of a novel abscisic acid-regulated farnesol dehydrogenase from Arabidopsis.Plant Physiol. 2010 Nov;154(3):1116-27. doi: 10.1104/pp.110.157784. Epub 2010 Aug 31. Plant Physiol. 2010. PMID: 20807998 Free PMC article.
-
Identification and functional expression in yeast of a prenylcysteine alpha-carboxyl methyltransferase gene from Arabidopsis thaliana.Plant Mol Biol. 2001 Mar;45(4):469-76. doi: 10.1023/a:1010671202925. Plant Mol Biol. 2001. PMID: 11352465
-
Protein prenylation in plants: old friends and new targets.Plant Mol Biol. 1999 Mar;39(5):865-70. doi: 10.1023/a:1006170020836. Plant Mol Biol. 1999. PMID: 10344192 Review. No abstract available.
-
Protein geranylgeranyltransferase I is involved in specific aspects of abscisic acid and auxin signaling in Arabidopsis.Plant Physiol. 2005 Oct;139(2):722-33. doi: 10.1104/pp.105.065045. Epub 2005 Sep 23. Plant Physiol. 2005. PMID: 16183844 Free PMC article.
-
Characterization, sub-cellular localization and expression profiling of the isoprenylcysteine methylesterase gene family in Arabidopsis thaliana.BMC Plant Biol. 2010 Sep 27;10:212. doi: 10.1186/1471-2229-10-212. BMC Plant Biol. 2010. PMID: 20868530 Free PMC article.
References
-
- Beranger F, Cadwallader K, Porfiri E, Powers S, Evans T, de Gunzberg J, Hancock JF. Determination of structural requirements for the interaction of Rab6 with RabGDI and Rab geranylgeranyltransferase. J Biol Chem. 1994;269:13637–13643. - PubMed
-
- Biermann BJ, Morehead TA, Tate SE, Price JR, Randall SK, Crowell DN. Novel isoprenylated proteins identified by an expression library screen. J Biol Chem. 1994;269:25251–25254. - PubMed
-
- Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. - PubMed
-
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

