N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells
- PMID: 9729617
- PMCID: PMC2231194
- DOI: 10.1111/j.1469-7793.1998.061bf.x
N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells
Abstract
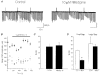
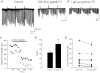
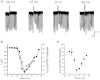
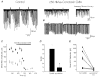
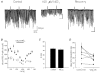
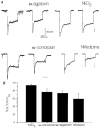
1. We investigated depolarization-induced suppression of inhibition (DSI) under whole-cell voltage clamp in CA1 pyramidal neurons of rat hippocampal slices. DSI, a transient reduction in monosynaptic evoked GABAAergic IPSCs lasting for approximately 1 min, was induced by depolarizing the pyramidal cell to -10 or 0 mV for 1 or 2 s. 2. Raising extracellular Ca2+ concentration increased DSI, and varying the DSI-inducing voltage step showed that the voltage dependence of DSI was like that of high-voltage-activated Ca2+ channels. 3. The P- and Q-type Ca2+ channel blocker omega-agatoxin TK (200 nM and 1 microM) and the R- and T-type Ca2+ channel blocker Ni2+ (100 microM) reduced IPSCs without reducing DSI. 4. The specific N-type Ca2+ channel antagonist omega-conotoxin GVIA (250 nM) reduced IPSC amplitudes and almost completely abolished DSI. 5. Blocking L-type Ca2+ channels with nifedipine (10 microM) had no effect on IPSCs or DSI induced by our standard protocol, but reduced DSI induced by the unclamped Na+- and Ca2+-dependent spikes that occurred when 2(triethylamino)-N-(2,6-dimethylphenyl)acetamide (QX-314) was omitted from the recording pipette solution. 6. Although intracellular Ca2+ stores were not measured, DSI was not affected by cyclopiazonic acid (CPA, 20-40 microM), a blocker of Ca2+ uptake into intracellular stores. 7. We conclude that DSI is initiated by Ca2+ influx through N- and, under certain conditions, L-type Ca2+ channels.
Figures
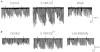
Similar articles
-
Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells.J Physiol. 1996 Oct 1;496 ( Pt 1)(Pt 1):197-209. doi: 10.1113/jphysiol.1996.sp021677. J Physiol. 1996. PMID: 8910208 Free PMC article.
-
Properties of calcium spikes revealed during GABAA receptor antagonism in hippocampal CA1 neurons from guinea pigs.J Neurophysiol. 1997 Nov;78(5):2269-79. doi: 10.1152/jn.1997.78.5.2269. J Neurophysiol. 1997. PMID: 9356380
-
Calcium channel currents in acutely dissociated intracardiac neurons from adult rats.J Neurophysiol. 1997 Apr;77(4):1769-78. doi: 10.1152/jn.1997.77.4.1769. J Neurophysiol. 1997. PMID: 9114235
-
Calcium, calcium channels, and calcium channel antagonists.Can J Physiol Pharmacol. 1990 Nov;68(11):1474-81. doi: 10.1139/y90-224. Can J Physiol Pharmacol. 1990. PMID: 1962736 Review.
-
Widening potential for Ca2+ antagonists: non-L-type Ca2+ channel interaction.Trends Pharmacol Sci. 1990 Jan;11(1):38-44. doi: 10.1016/0165-6147(90)90040-f. Trends Pharmacol Sci. 1990. PMID: 2155497 Review.
Cited by
-
Firing of hippocampal neurogliaform cells induces suppression of synaptic inhibition.J Neurosci. 2014 Jan 22;34(4):1280-92. doi: 10.1523/JNEUROSCI.3046-13.2014. J Neurosci. 2014. PMID: 24453319 Free PMC article.
-
Cannabinoid-dependent potentiation of inhibition at eye opening in mouse V1.Front Cell Neurosci. 2014 Feb 19;8:46. doi: 10.3389/fncel.2014.00046. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24600349 Free PMC article.
-
Ifenprodil, a NR2B-selective antagonist of NMDA receptor, inhibits reverse Na+/Ca2+ exchanger in neurons.Neuropharmacology. 2012 Nov;63(6):974-82. doi: 10.1016/j.neuropharm.2012.07.012. Epub 2012 Jul 20. Neuropharmacology. 2012. PMID: 22820271 Free PMC article.
-
Developmental alteration of endocannabinoid retrograde signaling in the hippocampus.J Neurophysiol. 2010 Feb;103(2):1123-9. doi: 10.1152/jn.00327.2009. Epub 2009 Dec 9. J Neurophysiol. 2010. PMID: 20007500 Free PMC article.
-
Metabotropic suppression of excitation in murine autaptic hippocampal neurons.J Physiol. 2007 Feb 1;578(Pt 3):773-85. doi: 10.1113/jphysiol.2006.117499. Epub 2006 Nov 16. J Physiol. 2007. PMID: 17110416 Free PMC article.
References
-
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends in Neurosciences. 1995;18:333–340. 10.1016/0166-2236(95)93923-L. - DOI - PubMed
-
- Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. European Journal of Pharmacology. 1991;199:259–262. 10.1016/0014-2999(91)90467-5. - DOI - PubMed
-
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

