Differential distribution of dynamin isoforms in mammalian cells
- PMID: 9725914
- PMCID: PMC25532
- DOI: 10.1091/mbc.9.9.2595
Differential distribution of dynamin isoforms in mammalian cells
Abstract
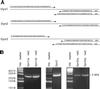
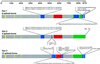
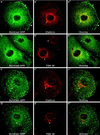
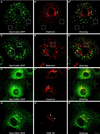
Dynamins are 100-kDa GTPases that are essential for clathrin-coated vesicle formation during receptor-mediated endocytosis. To date, three different dynamin genes have been identified, with each gene expressing at least four different alternatively spliced forms. Currently, it is unclear whether these different dynamin gene products perform distinct or redundant cellular functions. Therefore, the focus of this study was to identify additional spliced variants of dynamin from rat tissues and to define the distribution of the dynamin family members in a cultured rat epithelial cell model (Clone 9 cells). After long-distance reverse transcription (RT)-PCR of mRNA from different rat tissues, the full-length cDNAs encoding the different dynamin isoforms were sequenced and revealed four additional spliced variants for dynamin I and nine for dynamin III. Thus, in rat tissues there are a total of at least 25 different mRNAs produced from the three dynamin genes. Subsequently, we generated stably transfected Clone 9 cells expressing full-length cDNAs of six different spliced forms tagged with green fluorescent protein. Confocal or fluorescence microscopy of these transfected cells revealed that many of the dynamin proteins associate with distinct membrane compartments, which include clathrin-coated pits at the plasma membrane and the Golgi apparatus, and several undefined vesicle populations. These results indicate that the dynamin family is more extensive than was originally predicted and suggest that the different dynamin proteins are localized to distinct cytoplasmic or membrane compartments.
Figures


Similar articles
-
Association of a dynamin-like protein with the Golgi apparatus in mammalian cells.J Cell Biol. 1996 May;133(4):761-75. doi: 10.1083/jcb.133.4.761. J Cell Biol. 1996. PMID: 8666662 Free PMC article.
-
Dynamin-mediated internalization of caveolae.J Cell Biol. 1998 Apr 6;141(1):85-99. doi: 10.1083/jcb.141.1.85. J Cell Biol. 1998. PMID: 9531550 Free PMC article.
-
Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin.J Cell Sci. 2000 Jun;113 ( Pt 11):1993-2002. doi: 10.1242/jcs.113.11.1993. J Cell Sci. 2000. PMID: 10806110
-
Participation of dynamin in the biogenesis of cytoplasmic vesicles.FASEB J. 1999 Dec;13 Suppl 2:S243-7. doi: 10.1096/fasebj.13.9002.s243. FASEB J. 1999. PMID: 10619136 Review.
-
Dynamin, a membrane-remodelling GTPase.Nat Rev Mol Cell Biol. 2012 Jan 11;13(2):75-88. doi: 10.1038/nrm3266. Nat Rev Mol Cell Biol. 2012. PMID: 22233676 Free PMC article. Review.
Cited by
-
Structural insights into the centronuclear myopathy-associated functions of BIN1 and dynamin 2.J Struct Biol. 2016 Oct;196(1):37-47. doi: 10.1016/j.jsb.2016.06.015. Epub 2016 Jun 23. J Struct Biol. 2016. PMID: 27343996 Free PMC article. Review.
-
Dynamin 1- and 3-Mediated Endocytosis Is Essential for the Development of a Large Central Synapse In Vivo.J Neurosci. 2016 Jun 1;36(22):6097-115. doi: 10.1523/JNEUROSCI.3804-15.2016. J Neurosci. 2016. PMID: 27251629 Free PMC article.
-
Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer.Neuron. 2007 Sep 20;55(6):874-89. doi: 10.1016/j.neuron.2007.06.041. Neuron. 2007. PMID: 17880892 Free PMC article.
-
Dynamin GTPase regulation is altered by PH domain mutations found in centronuclear myopathy patients.EMBO J. 2010 Sep 15;29(18):3054-67. doi: 10.1038/emboj.2010.187. Epub 2010 Aug 10. EMBO J. 2010. PMID: 20700106 Free PMC article.
-
Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles.Mol Biol Cell. 2002 Jan;13(1):169-82. doi: 10.1091/mbc.01-07-0380. Mol Biol Cell. 2002. PMID: 11809831 Free PMC article.
References
-
- Chen MS, Burgess CC, Vallee RB, Wadsworth SC. Developmental stage- and tissue-specific expression of shibire, a Drosophila gene involved in endocytosis. J Cell Sci. 1992;103:619–628. - PubMed
-
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem, 1987;162:156–159. - PubMed
-
- Conti AM, Fischer SJ, Windebank AJ. Inhibition of axonal growth from sensory neurons by excess nerve growth factor. Ann Neurol. 1997;42:838–846. - PubMed
-
- Cook T, Mesa K, Urrutia R. Three dynamin-encoding genes are differently expressed in developing rat brain. J Neurochem. 1996;67:927–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

