Mode of action of (1'S,2'R)-9-[[1',2'-bis(hydroxymethyl) cycloprop-1'-yl]methyl]guanine (A-5021) against herpes simplex virus type 1 and type 2 and varicella-zoster virus
- PMID: 9687413
- PMCID: PMC105870
- DOI: 10.1128/AAC.42.8.2095
Mode of action of (1'S,2'R)-9-[[1',2'-bis(hydroxymethyl) cycloprop-1'-yl]methyl]guanine (A-5021) against herpes simplex virus type 1 and type 2 and varicella-zoster virus
Abstract
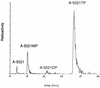
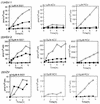
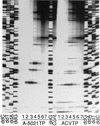
The mode of action of (1'S,2'R)-9-([1', 2'-bis(hydroxymethyl)cycloprop-1'-yl]methyl)guanine (A-5021) against herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus (VZV) was studied. A-5021 was monophosphorylated at the 2' site by viral thymidine kinases (TKs). The 50% inhibitory values for thymidine phosphorylation of A-5021 by HSV-1 TK and HSV-2 TK were comparable to those for penciclovir (PCV) and lower than those for acyclovir (ACV). Of these three agents, A-5021 inhibited VZV TK most efficiently. A-5021 was phosphorylated to a mono-, di-, and triphosphate in MRC-5 cells infected with HSV-1, HSV-2, and VZV. A-5021 triphosphate accumulated more than ACV triphosphate but less than PCV triphosphate in MRC-5 cells infected with HSV-1 or VZV, whereas HSV-2-infected MRC-5 cells had comparable levels of A-5021 and ACV triphosphates. The intracellular half-life of A-5021 triphosphate was considerably longer than that of ACV triphosphate and shorter than that of PCV triphosphate. A-5021 triphosphate competitively inhibited HSV DNA polymerases with respect to dGTP. Inhibition was strongest with ACV triphosphate, followed by A-5021 triphosphate and then (R,S)-PCV triphosphate. A DNA chain elongation experiment revealed that A-5021 triphosphate was incorporated into DNA instead of dGTP and terminated elongation, although limited chain extension was observed. Thus, the strong antiviral activity of A-5021 appears to depend on a more rapid and stable accumulation of its triphosphate in infected cells than that of ACV and on stronger inhibition of viral DNA polymerase by its triphosphate than that of PCV.
Figures

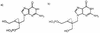


Similar articles
-
Antiherpesvirus activities of (1'S,2'R)-9-[[1',2'-bis(hydroxymethyl)cycloprop-1'-yl]methyl]guanine (A-5021) in cell culture.Antimicrob Agents Chemother. 1998 Jul;42(7):1666-70. doi: 10.1128/AAC.42.7.1666. Antimicrob Agents Chemother. 1998. PMID: 9661001 Free PMC article.
-
Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses.Antimicrob Agents Chemother. 1995 Aug;39(8):1802-8. doi: 10.1128/AAC.39.8.1802. Antimicrob Agents Chemother. 1995. PMID: 7486922 Free PMC article.
-
Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus.Antimicrob Agents Chemother. 1992 Dec;36(12):2747-57. doi: 10.1128/AAC.36.12.2747. Antimicrob Agents Chemother. 1992. PMID: 1336346 Free PMC article.
-
Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management.Antimicrob Agents Chemother. 2011 Feb;55(2):459-72. doi: 10.1128/AAC.00615-10. Epub 2010 Nov 15. Antimicrob Agents Chemother. 2011. PMID: 21078929 Free PMC article. Review.
-
[Anti alpha-herpesvirus drugs].Nihon Rinsho. 2012 Apr;70(4):558-63. Nihon Rinsho. 2012. PMID: 22568134 Review. Japanese.
Cited by
-
Distribution and effects of amino acid changes in drug-resistant α and β herpesviruses DNA polymerase.Nucleic Acids Res. 2016 Nov 16;44(20):9530-9554. doi: 10.1093/nar/gkw875. Epub 2016 Sep 29. Nucleic Acids Res. 2016. PMID: 27694307 Free PMC article. Review.
-
A new nucleoside analogue with potent activity against mutant sr39 herpes simplex virus-1 (HSV-1) thymidine kinase (TK).Org Lett. 2012 Jul 20;14(14):3568-71. doi: 10.1021/ol300728a. Epub 2012 Jul 5. Org Lett. 2012. PMID: 22765027 Free PMC article.
-
The next ten stories on antiviral drug discovery (part E): advents, advances, and adventures.Med Res Rev. 2011 Jan;31(1):118-60. doi: 10.1002/med.20179. Med Res Rev. 2011. PMID: 19844936 Free PMC article. Review.
-
Antiherpesvirus activities of (1'S,2'R)-9-[[1',2'-bis(hydroxymethyl)cycloprop-1'-yl]methyl]guanine (A-5021) in cell culture.Antimicrob Agents Chemother. 1998 Jul;42(7):1666-70. doi: 10.1128/AAC.42.7.1666. Antimicrob Agents Chemother. 1998. PMID: 9661001 Free PMC article.
References
-
- Abele G, Cox S, Bergman S, Lindbergh N, Vissgarden A, Karlstorm A, Harmenberg J, Wahren B. Antiviral activity against VZV and HSV type 1 and type 2 of the (+) and (−) enantiomers of (R,S)-9-/4-hydroxy-2-(hydroxymethyl)butyl/guanine, in comparison to other closely related acyclic nucleosides. Antivir Chem Chemother. 1991;2:163–169.
-
- Bacon T H, Gilbart J, Howard B A, Standring-Cox R. Inhibition of varicella-zoster virus by penciclovir in cell culture and mechanism of action. Antivir Chem Chemother. 1996;7:71–78.
-
- Cheng Y C, Ostrander M. Deoxythymidine kinase induced in HeLa TK− cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976;251:2605–2610. - PubMed
-
- Darby, G. 1994. A history of antiherpes research. Antivir. Chem. Chemother. 5(Suppl. 1):3–9.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

