Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells
- PMID: 9653172
- PMCID: PMC20961
- DOI: 10.1073/pnas.95.14.8245
Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells
Abstract
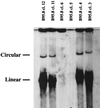
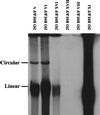
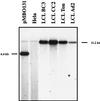
With current techniques, genetic alterations of herpesviruses are difficult to perform, mostly because of the large size of their genomes. To solve this problem, we have designed a system that allows the cloning of any gamma-herpesvirus in Escherichia coli onto an F factor-derived plasmid. Immortalized B cell lines were readily established with recombinant Epstein-Barr virus (EBV), demonstrating that the F factor-cloned EBV genome has all the characteristics of wild-type EBV. Because any genetic modification is possible in E. coli, this experimental approach opens the way to the genetic analysis of all EBV functions. Moreover, it is now feasible to generate attenuated EBV strains in vitro such that vaccine strains can be designed. Because we incorporated the genes for hygromycin resistance and green fluorescent protein onto the E. coli cloned EBV genome, the still open question of the EBV target cells other than B lymphocytes will be addressed.
Figures


Similar articles
-
The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization.J Virol. 2020 Aug 17;94(17):e01215-20. doi: 10.1128/JVI.01215-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581094 Free PMC article.
-
Epstein-Barr virus as an agent of haematological disease.Baillieres Clin Haematol. 1995 Mar;8(1):165-99. doi: 10.1016/s0950-3536(05)80237-9. Baillieres Clin Haematol. 1995. PMID: 7663046 Review.
-
Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus.J Virol. 1999 Apr;73(4):2974-82. doi: 10.1128/JVI.73.4.2974-2982.1999. J Virol. 1999. PMID: 10074147 Free PMC article.
-
Epstein-Barr virus and the B cell: that's all it takes.Trends Microbiol. 1996 May;4(5):204-8. doi: 10.1016/s0966-842x(96)90020-7. Trends Microbiol. 1996. PMID: 8727601 Review.
-
Heterogeneous expression of Epstein-Barr virus latent proteins in endemic Burkitt's lymphoma.Blood. 1995 Jul 15;86(2):659-65. Blood. 1995. PMID: 7605996
Cited by
-
Ubiquitin Modification of the Epstein-Barr Virus Immediate Early Transactivator Zta.J Virol. 2020 Oct 27;94(22):e01298-20. doi: 10.1128/JVI.01298-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847852 Free PMC article.
-
Induction of p16(INK4a) is the major barrier to proliferation when Epstein-Barr virus (EBV) transforms primary B cells into lymphoblastoid cell lines.PLoS Pathog. 2013 Feb;9(2):e1003187. doi: 10.1371/journal.ppat.1003187. Epub 2013 Feb 21. PLoS Pathog. 2013. PMID: 23436997 Free PMC article.
-
DNA Damage Signaling Is Induced in the Absence of Epstein-Barr Virus (EBV) Lytic DNA Replication and in Response to Expression of ZEBRA.PLoS One. 2015 May 7;10(5):e0126088. doi: 10.1371/journal.pone.0126088. eCollection 2015. PLoS One. 2015. PMID: 25950714 Free PMC article.
-
The EBNA2 polyproline region is dispensable for Epstein-Barr virus-mediated immortalization maintenance.J Virol. 2002 Jul;76(14):7349-55. doi: 10.1128/jvi.76.14.7349-7355.2002. J Virol. 2002. PMID: 12072534 Free PMC article.
-
Cloning the vaccinia virus genome as a bacterial artificial chromosome in Escherichia coli and recovery of infectious virus in mammalian cells.Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12415-20. doi: 10.1073/pnas.192420599. Epub 2002 Aug 26. Proc Natl Acad Sci U S A. 2002. PMID: 12196634 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

