Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells
- PMID: 9621012
- PMCID: PMC110204
- DOI: 10.1128/JVI.72.7.5552-5558.1998
Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells
Abstract
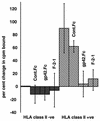
The Epstein-Barr virus (EBV) gH-gL complex includes a third glycoprotein, gp42. gp42 binds to HLA class II on the surfaces of B lymphocytes, and this interaction is essential for infection of the B cell. We report here that, in contrast, gp42 is dispensable for infection of epithelial cell line SVKCR2. A soluble form of gp42, gp42.Fc, can, however, inhibit infection of both cell types. Soluble gp42 can interact with EBV gH and gL and can rescue the ability of virus lacking gp42 to transform B cells, suggesting that a gH-gL-gp42.Fc complex can be formed by extrinsic addition of the soluble protein. Truncated forms of gp42.Fc that retain the ability to bind HLA class II but that cannot interact with gH and gL still inhibit B-cell infection by wild-type virus but cannot inhibit infection of SVKCR2 cells or rescue the ability of recombinant gp42-negative virus to transform B cells. An analysis of wild-type virions indicates the presence of more gH and gL than gp42. To explain these results, we describe a model in which wild-type EBV virions are proposed to contain two types of gH-gL complexes, one that includes gp42 and one that does not. We further propose that these two forms of the complex have mutually exclusive abilities to mediate the infection of B cells and epithelial cells. Conversion of one to the other concurrently alters the ability of virus to infect each cell type. The model also suggests that epithelial cells may express a molecule that serves the same cofactor function for this cell type as HLA class II does for B cells and that the gH-gL complex interacts directly with this putative epithelial cofactor.
Figures






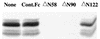

Similar articles
-
Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion.J Virol. 2006 Oct;80(19):9444-54. doi: 10.1128/JVI.00572-06. J Virol. 2006. PMID: 16973550 Free PMC article.
-
Binding-site interactions between Epstein-Barr virus fusion proteins gp42 and gH/gL reveal a peptide that inhibits both epithelial and B-cell membrane fusion.J Virol. 2007 Sep;81(17):9216-29. doi: 10.1128/JVI.00575-07. Epub 2007 Jun 20. J Virol. 2007. PMID: 17581996 Free PMC article.
-
Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells.J Virol. 2000 Jul;74(14):6324-32. doi: 10.1128/jvi.74.14.6324-6332.2000. J Virol. 2000. PMID: 10864642 Free PMC article.
-
Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus.Mol Cells. 2016 Apr 30;39(4):286-91. doi: 10.14348/molcells.2016.0066. Epub 2016 Apr 6. Mol Cells. 2016. PMID: 27094060 Free PMC article. Review.
-
[The entry of Epstein-Barr virus into B lymphocytes and epithelial cells during infection].Bing Du Xue Bao. 2014 Jul;30(4):476-82. Bing Du Xue Bao. 2014. PMID: 25272606 Review. Chinese.
Cited by
-
Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion.J Virol. 2006 Mar;80(5):2216-24. doi: 10.1128/JVI.80.5.2216-2224.2006. J Virol. 2006. PMID: 16474129 Free PMC article.
-
The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates.J Virol. 2002 Nov;76(21):10841-8. doi: 10.1128/jvi.76.21.10841-10848.2002. J Virol. 2002. PMID: 12368327 Free PMC article.
-
Cell-surface expression of a mutated Epstein-Barr virus glycoprotein B allows fusion independent of other viral proteins.Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17474-9. doi: 10.1073/pnas.0404535101. Epub 2004 Dec 6. Proc Natl Acad Sci U S A. 2004. PMID: 15583133 Free PMC article.
-
Gamma herpesviruses: pathogenesis of infection and cell signaling.Folia Microbiol (Praha). 2003;48(3):291-318. doi: 10.1007/BF02931360. Folia Microbiol (Praha). 2003. PMID: 12879740 Review.
-
Cellular and viral requirements for rapid endocytic entry of herpes simplex virus.J Virol. 2004 Jul;78(14):7508-17. doi: 10.1128/JVI.78.14.7508-7517.2004. J Virol. 2004. PMID: 15220424 Free PMC article.
References
-
- Borza, C., and L. M. Hutt-Fletcher. Unpublished data.
-
- Duus K M, Hatfield C, Grose C. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology. 1995;210:429–440. - PubMed
-
- Fanslow W C, Anderson D M, Grabstein K H, Clark E A, Cosman D, Armitage R J. Soluble forms of CD40 inhibit biologic responses of human B cells. J Immunol. 1992;149:655–660. - PubMed
-
- Forghani B, Ni L, Grose C. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology. 1994;199:458–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

