A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells
- PMID: 9614185
- PMCID: PMC25366
- DOI: 10.1091/mbc.9.6.1437
A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells
Abstract
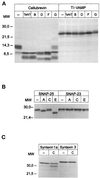
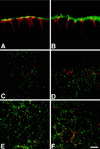
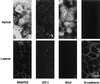
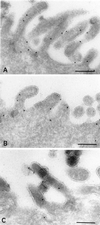
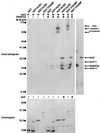
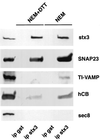
The importance of soluble N-ethyl maleimide (NEM)-sensitive fusion protein (NSF) attachment protein (SNAP) receptors (SNAREs) in synaptic vesicle exocytosis is well established because it has been demonstrated that clostridial neurotoxins (NTs) proteolyze the vesicle SNAREs (v-SNAREs) vesicle-associated membrane protein (VAMP)/brevins and their partners, the target SNAREs (t-SNAREs) syntaxin 1 and SNAP25. Yet, several exocytotic events, including apical exocytosis in epithelial cells, are insensitive to numerous clostridial NTs, suggesting the presence of SNARE-independent mechanisms of exocytosis. In this study we found that syntaxin 3, SNAP23, and a newly identified VAMP/brevin, tetanus neurotoxin (TeNT)-insensitive VAMP (TI-VAMP), are insensitive to clostridial NTs. In epithelial cells, TI-VAMP-containing vesicles were concentrated in the apical domain, and the protein was detected at the apical plasma membrane by immunogold labeling on ultrathin cryosections. Syntaxin 3 and SNAP23 were codistributed at the apical plasma membrane where they formed NEM-dependent SNARE complexes with TI-VAMP and cellubrevin. We suggest that TI-VAMP, SNAP23, and syntaxin 3 can participate in exocytotic processes at the apical plasma membrane of epithelial cells and, more generally, domain-specific exocytosis in clostridial NT-resistant pathways.
Figures

Similar articles
-
Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth.J Cell Biol. 2000 May 15;149(4):889-900. doi: 10.1083/jcb.149.4.889. J Cell Biol. 2000. PMID: 10811829 Free PMC article.
-
Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment.J Immunol. 2000 Jun 1;164(11):5850-7. doi: 10.4049/jimmunol.164.11.5850. J Immunol. 2000. PMID: 10820264
-
The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways?Traffic. 2005 May;6(5):366-73. doi: 10.1111/j.1600-0854.2005.00288.x. Traffic. 2005. PMID: 15813747 Review.
-
Ectopic expression of syntaxin 1 in the ER redirects TI-VAMP- and cellubrevin-containing vesicles.J Cell Sci. 2003 Jul 1;116(Pt 13):2805-16. doi: 10.1242/jcs.00467. Epub 2003 May 20. J Cell Sci. 2003. PMID: 12759369
-
[Analysis of synaptic neurotransmitter release mechanisms using bacterial toxins].J Soc Biol. 1999;193(6):457-67. J Soc Biol. 1999. PMID: 10783704 Review. French.
Cited by
-
Role of tetanus neurotoxin insensitive vesicle-associated membrane protein in membrane domains transport and homeostasis.Cell Logist. 2015 Apr 29;5(1):e1025182. doi: 10.1080/21592799.2015.1025182. eCollection 2015 Jan-Mar. Cell Logist. 2015. PMID: 26196023 Free PMC article.
-
VAMP2 is implicated in the secretion of antibodies by human plasma cells and can be replaced by other synaptobrevins.Cell Mol Immunol. 2018 Apr;15(4):353-366. doi: 10.1038/cmi.2016.46. Epub 2016 Sep 12. Cell Mol Immunol. 2018. PMID: 27616736 Free PMC article.
-
Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: processes that resemble terminal differentiation.J Cell Biol. 1999 Mar 8;144(5):1057-67. doi: 10.1083/jcb.144.5.1057. J Cell Biol. 1999. PMID: 10085301 Free PMC article.
-
Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3.J Cell Biol. 2015 Nov 9;211(3):587-604. doi: 10.1083/jcb.201506112. J Cell Biol. 2015. PMID: 26553929 Free PMC article.
-
Characterization of VAMP-2 in the lung: implication in lung surfactant secretion.Cell Biol Int. 2012 Sep;36(9):785-91. doi: 10.1042/CBI20110146. Cell Biol Int. 2012. PMID: 22571236 Free PMC article.
References
-
- Banerjee A, Barry VA, DasGupta BR, Martin TFJ. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. - PubMed
-
- Beckers CJ, Block MR, Glick BS, Rothman JE, Balch WE. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989;339:397–398. - PubMed
-
- Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. - PubMed
-
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993a;365:160–163. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

