Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains
- PMID: 9585410
- PMCID: PMC2132770
- DOI: 10.1083/jcb.141.4.905
Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains
Abstract
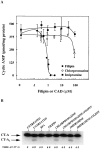
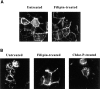
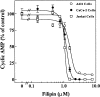
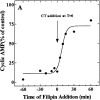
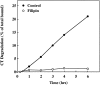
The mechanism by which cholera toxin (CT) is internalized from the plasma membrane before its intracellular reduction and subsequent activation of adenylyl cyclase is not well understood. Ganglioside GM1, the receptor for CT, is predominantly clustered in detergent-insoluble glycolipid rafts and in caveolae, noncoated, cholesterol-rich invaginations on the plasma membrane. In this study, we used filipin, a sterol-binding agent that disrupts caveolae and caveolae-like structures, to explore their role in the internalization and activation of CT in CaCo-2 human intestinal epithelial cells. When toxin internalization was quantified, only 33% of surface-bound toxin was internalized by filipin-treated cells within 1 h compared with 79% in untreated cells. However, CT activation as determined by its reduction to form the A1 peptide and CT activity as measured by cyclic AMP accumulation were inhibited in filipin-treated cells. Another sterol-binding agent, 2-hydroxy-beta-cyclodextrin, gave comparable results. The cationic amphiphilic drug chlorpromazine, an inhibitor of clathrin-dependent, receptor-mediated endocytosis, however, affected neither CT internalization, activation, nor activity in contrast to its inhibitory effects on diphtheria toxin cytotoxicity. As filipin did not inhibit the latter, the two drugs appeared to distinguish between caveolae- and coated pit-mediated processes. In addition to its effects in CaCo-2 cells that express low levels of caveolin, filipin also inhibited CT activity in human epidermoid carcinoma A431 and Jurkat T lymphoma cells that are, respectively, rich in or lack caveolin. Thus, filipin inhibition correlated more closely with alterations in the biochemical characteristics of CT-bound membranes due to the interactions of filipin with cholesterol rather than with the expressed levels of caveolin and caveolar structure. Our results indicated that the internalization and activation of CT was dependent on and mediated through cholesterol- and glycolipid-rich microdomains at the plasma membrane rather than through a specific morphological structure and that these glycolipid microdomains have the necessary components required to mediate endocytosis.
Figures
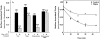

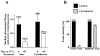


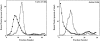
Similar articles
-
Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action.J Biol Chem. 1993 Jun 5;268(16):12010-6. J Biol Chem. 1993. PMID: 8389369
-
Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia.J Cell Biol. 1998 May 18;141(4):917-27. doi: 10.1083/jcb.141.4.917. J Cell Biol. 1998. PMID: 9585411 Free PMC article.
-
Internalization of cholera toxin by different endocytic mechanisms.J Cell Sci. 2001 Oct;114(Pt 20):3737-47. doi: 10.1242/jcs.114.20.3737. J Cell Sci. 2001. PMID: 11707525
-
Floating cholera toxin into epithelial cells: functional association with caveolae-like detergent-insoluble membrane microdomains.Int J Med Microbiol. 2000 Oct;290(4-5):403-8. doi: 10.1016/S1438-4221(00)80052-1. Int J Med Microbiol. 2000. PMID: 11111918 Review.
-
Caveolae--an alternative endocytotic pathway for targeted drug delivery.Crit Rev Ther Drug Carrier Syst. 2004;21(2):67-95. doi: 10.1615/critrevtherdrugcarriersyst.v21.i2.10. Crit Rev Ther Drug Carrier Syst. 2004. PMID: 15202927 Review.
Cited by
-
Visualizing Coronavirus Entry into Cells.Methods Mol Biol. 2020;2203:241-261. doi: 10.1007/978-1-0716-0900-2_18. Methods Mol Biol. 2020. PMID: 32833217
-
Cellular Endocytosis and Trafficking of Cholera Toxin B-Modified Mesoporous Silica Nanoparticles.J Mater Chem B. 2016 Feb 21;4(7):1254-1262. doi: 10.1039/C5TB02079D. Epub 2016 Jan 7. J Mater Chem B. 2016. PMID: 27134749 Free PMC article.
-
Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine.Chem Soc Rev. 2021 May 7;50(9):5397-5434. doi: 10.1039/d0cs01127d. Epub 2021 Mar 5. Chem Soc Rev. 2021. PMID: 33666625 Free PMC article. Review.
-
The conserved metalloprotease invadolysin localizes to the surface of lipid droplets.J Cell Sci. 2009 Sep 15;122(Pt 18):3414-23. doi: 10.1242/jcs.044610. Epub 2009 Aug 25. J Cell Sci. 2009. PMID: 19706689 Free PMC article.
-
Endocytic mechanisms for targeted drug delivery.Adv Drug Deliv Rev. 2007 Aug 10;59(8):748-58. doi: 10.1016/j.addr.2007.06.008. Epub 2007 Jun 28. Adv Drug Deliv Rev. 2007. PMID: 17659804 Free PMC article. Review.
References
-
- Anderson RGW, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
-
- Beaumelle B, Bensammar L, Bienvenüe A. Selective translocation of the A chain of diphtheria toxin across the membrane of purified endosomes. J Biol Chem. 1992;267:11525–11531. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical surface. Cell. 1992;68:533–544. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

