Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis
- PMID: 9573052
- PMCID: PMC316787
- DOI: 10.1101/gad.12.9.1356
Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis
Abstract
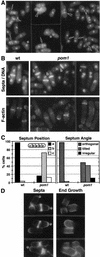
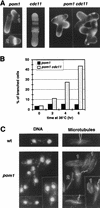
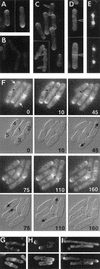
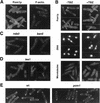
Schizosaccharomyces pombe cells have a well-defined pattern of polarized growth at the cell ends during interphase and divide symmetrically into two equal-sized daughter cells. We identified a gene, pom1, that provides positional information for both growth and division in S. pombe. pom1 mutants form functioning growth zones and division septa but show several abnormalities: (1) After division, cells initiate growth with equal frequencies from either the old or the new end; (2) most cells never switch to bipolar growth but instead grow exclusively at the randomly chosen end; (3) some cells mislocalize their growth axis altogether, leading to the formation of angled and branched cells; and (4) many cells misplace and/or misorient their septa, leading to asymmetric cell division. pom1 encodes a putative protein kinase that is concentrated at the new cell end during interphase, at both cell ends during mitosis, and at the septation site after mitosis. Small amounts of Pom1p are also found at the old cell end during interphase and associated with the actin ring during mitosis. Pom1p localization to the cell ends is independent of actin but requires microtubules and Tea1p. pom1 mutations are synthetically lethal with several other mutations that affect cytokinesis and/or the actin or microtubule cytoskeleton. Thus, Pom1p may position the growth and cytokinesis machineries by interaction with both the actin and microtubule cytoskeletons.
Figures



Similar articles
-
Role of Tea1p, Tea3p and Pom1p in the determination of cell ends in Schizosaccharomyces pombe.Yeast. 2003 Dec;20(16):1349-58. doi: 10.1002/yea.1054. Yeast. 2003. PMID: 14663827
-
Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast.Curr Biol. 2001 Jun 5;11(11):836-45. doi: 10.1016/s0960-9822(01)00235-4. Curr Biol. 2001. PMID: 11516644
-
Spatial regulation of cytokinesis by the Kin1 and Pom1 kinases in fission yeast.Curr Genet. 2006 Dec;50(6):377-91. doi: 10.1007/s00294-006-0099-5. Epub 2006 Sep 20. Curr Genet. 2006. PMID: 16988828
-
Establishment of a cellular axis in fission yeast.Trends Genet. 2001 May;17(5):273-8. doi: 10.1016/s0168-9525(01)02279-x. Trends Genet. 2001. PMID: 11335037 Review.
-
Studies in fission yeast on mechanisms of cell division site placement.Cell Struct Funct. 2001 Dec;26(6):539-44. doi: 10.1247/csf.26.539. Cell Struct Funct. 2001. PMID: 11942607 Review.
Cited by
-
Characterization of Mid1 domains for targeting and scaffolding in fission yeast cytokinesis.J Cell Sci. 2012 Jun 15;125(Pt 12):2973-85. doi: 10.1242/jcs.102574. Epub 2012 Mar 16. J Cell Sci. 2012. PMID: 22427686 Free PMC article.
-
The role of anillin/Mid1p during medial division and cytokinesis: from fission yeast to cancer cells.Cell Cycle. 2023 Mar-Mar;22(6):633-644. doi: 10.1080/15384101.2022.2147655. Epub 2022 Nov 25. Cell Cycle. 2023. PMID: 36426865 Free PMC article. Review.
-
Cytokinesis in eukaryotes.Microbiol Mol Biol Rev. 2002 Jun;66(2):155-78. doi: 10.1128/MMBR.66.2.155-178.2002. Microbiol Mol Biol Rev. 2002. PMID: 12040122 Free PMC article. Review.
-
Molecular mechanisms of contractile-ring constriction and membrane trafficking in cytokinesis.Biophys Rev. 2018 Dec;10(6):1649-1666. doi: 10.1007/s12551-018-0479-3. Epub 2018 Nov 17. Biophys Rev. 2018. PMID: 30448943 Free PMC article. Review.
-
Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe.Mol Biol Cell. 2005 Apr;16(4):2003-17. doi: 10.1091/mbc.e04-06-0442. Epub 2005 Feb 2. Mol Biol Cell. 2005. PMID: 15689498 Free PMC article.
References
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993.
-
- Bähler, J., J.-Q. Wu, M.S. Longtine, N.G. Shah, A. McKenzie III, A.B. Steever, A. Wach, P. Philippsen, and J.R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast (in press). - PubMed
-
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
