Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC
- PMID: 9570783
- PMCID: PMC6793167
- DOI: 10.1523/JNEUROSCI.18-10-03521.1998
Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC
Abstract
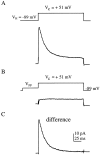
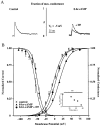
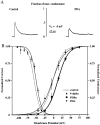
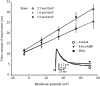
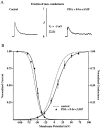
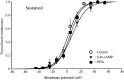
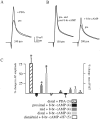
We have reported recently a high density of transient A-type K+ channels located in the distal dendrites of CA1 hippocampal pyramidal neurons and shown that these channels shape EPSPs, limit the back-propagation of action potentials, and prevent dendritic action potential initiation (). Because of the importance of these channels in dendritic signal propagation, their modulation by protein kinases would be of significant interest. We investigated the effects of activators of cAMP-dependent protein kinase (PKA) and the Ca2+-dependent phospholipid-sensitive protein kinase (PKC) on K+ channels in cell-attached patches from the distal dendrites of hippocampal CA1 pyramidal neurons. Inclusion of the membrane-permeant PKA activators 8-bromo-cAMP (8-br-cAMP) or forskolin in the dendritic patch pipette resulted in a depolarizing shift in the activation curve for the transient channels of approximately 15 mV. Activation of PKC by either of two phorbol esters also resulted in a 15 mV depolarizing shift of the activation curve. Neither PKA nor PKC activation affected the sustained or slowly inactivating component of the total outward current. This downregulation of transient K+ channels in the distal dendrites may be responsible for some of the frequently reported increases in cell excitability found after PKA and PKC activation. In support of this hypothesis, we found that activation of either PKA or PKC significantly increased the amplitude of back-propagating action potentials in distal dendrites.
Figures
Similar articles
-
Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway.J Neurosci. 2002 Jun 15;22(12):4860-8. doi: 10.1523/JNEUROSCI.22-12-04860.2002. J Neurosci. 2002. PMID: 12077183 Free PMC article.
-
Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K(+) channels in hippocampal CA1 pyramidal neurons.J Neurosci. 1999 Oct 1;19(19):8163-71. doi: 10.1523/JNEUROSCI.19-19-08163.1999. J Neurosci. 1999. PMID: 10493718 Free PMC article.
-
Dopamine D1/D5 receptor modulates state-dependent switching of soma-dendritic Ca2+ potentials via differential protein kinase A and C activation in rat prefrontal cortical neurons.J Neurosci. 2004 Jan 7;24(1):8-23. doi: 10.1523/JNEUROSCI.1650-03.2004. J Neurosci. 2004. PMID: 14715933 Free PMC article.
-
Dendritic potassium channels in hippocampal pyramidal neurons.J Physiol. 2000 May 15;525 Pt 1(Pt 1):75-81. doi: 10.1111/j.1469-7793.2000.00075.x. J Physiol. 2000. PMID: 10811726 Free PMC article. Review.
-
Electrical and calcium signaling in dendrites of hippocampal pyramidal neurons.Annu Rev Physiol. 1998;60:327-46. doi: 10.1146/annurev.physiol.60.1.327. Annu Rev Physiol. 1998. PMID: 9558467 Review.
Cited by
-
Protein kinase C bidirectionally modulates Ih and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel surface expression in hippocampal pyramidal neurons.J Physiol. 2015 Jul 1;593(13):2779-92. doi: 10.1113/JP270453. Epub 2015 May 22. J Physiol. 2015. PMID: 25820761 Free PMC article.
-
The spike-timing dependence of plasticity.Neuron. 2012 Aug 23;75(4):556-71. doi: 10.1016/j.neuron.2012.08.001. Neuron. 2012. PMID: 22920249 Free PMC article. Review.
-
Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons.Neuron. 2007 Jun 21;54(6):933-47. doi: 10.1016/j.neuron.2007.05.026. Neuron. 2007. PMID: 17582333 Free PMC article.
-
Presynaptic mechanism for phorbol ester-induced synaptic potentiation.J Neurosci. 1999 Sep 1;19(17):7262-7. doi: 10.1523/JNEUROSCI.19-17-07262.1999. J Neurosci. 1999. PMID: 10460232 Free PMC article.
-
Long-Term Inactivation of Sodium Channels as a Mechanism of Adaptation in CA1 Pyramidal Neurons.J Neurosci. 2022 May 4;42(18):3768-3782. doi: 10.1523/JNEUROSCI.1914-21.2022. Epub 2022 Mar 24. J Neurosci. 2022. PMID: 35332085 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Adams JP, Anderson AE, Johnston D, Pfaffinger PJ, Sweatt JD. Kv4.2: a novel substrate for MAP kinase phosphorylation. Soc Neurosci Abstr. 1997;23:1176.
-
- Alonso G, Widmer H. Clustering of Kv4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
-
- Anderson AE, Adams JP, Swann JW, Johnston D, Pfaffinger PJ, Sweatt JD. Kv4.2, a fast transient A-type potassium channel is a substrate for PKA and PKC. Soc Neurosci Abstr. 1997;23:1394.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
