Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes
- PMID: 9565631
- PMCID: PMC2212278
- DOI: 10.1084/jem.187.9.1383
Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes
Abstract
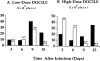
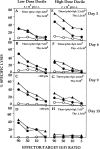
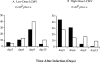
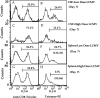
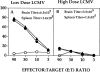
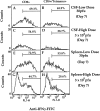
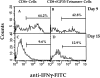
This study describes the construction of soluble major histocompatibility complexes consisting of the mouse class I molecule, H-2Db, chemically biotinylated beta2 microglobulin and a peptide epitope derived from the glycoprotein (GP; amino acids 33-41) of lymphocytic choriomeningitis virus (LCMV). Tetrameric class I complexes, which were produced by mixing the class I complexes with phycoerythrin-labeled neutravidin, permitted direct analysis of virus-specific cytotoxic T lymphocytes (CTLs) by flow cytometry. This technique was validated by (a) staining CD8+ cells in the spleens of transgenic mice that express a T cell receptor (TCR) specific for H-2Db in association with peptide GP33-41, and (b) by staining virus-specific CTLs in the cerebrospinal fluid of C57BL/6 (B6) mice that had been infected intracranially with LCMV-DOCILE. Staining of spleen cells isolated from B6 mice revealed that up to 40% of CD8(+) T cells were GP33 tetramer+ during the initial phase of LCMV infection. In contrast, GP33 tetramers did not stain CD8+ T cells isolated from the spleens of B6 mice that had been infected 2 mo previously with LCMV above the background levels found in naive mice. The fate of virus-specific CTLs was analyzed during the acute phase of infection in mice challenged both intracranially and intravenously with a high or low dose of LCMV-DOCILE. The results of the study show that the outcome of infection by LCMV is determined by antigen load alone. Furthermore, the data indicate that deletion of virus-specific CTLs in the presence of excessive antigen is preceded by TCR downregulation and is dependent upon perforin.
Figures





Similar articles
-
In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33-43 epitope by H-2Db but not H-2Kb.J Virol. 2001 Jun;75(11):5099-107. doi: 10.1128/JVI.75.11.5099-5107.2001. J Virol. 2001. PMID: 11333891 Free PMC article.
-
Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity.J Exp Med. 1996 May 1;183(5):2143-52. doi: 10.1084/jem.183.5.2143. J Exp Med. 1996. PMID: 8642324 Free PMC article.
-
Restricted V-segment usage in T-cell receptors from cytotoxic T lymphocytes specific for a major epitope of lymphocytic choriomeningitis virus.J Virol. 1990 Dec;64(12):5919-26. doi: 10.1128/JVI.64.12.5919-5926.1990. J Virol. 1990. PMID: 1700830 Free PMC article.
-
Viruses Teaching Immunology: Role of LCMV Model and Human Viral Infections in Immunological Discoveries.Viruses. 2019 Jan 27;11(2):106. doi: 10.3390/v11020106. Viruses. 2019. PMID: 30691215 Free PMC article. Review.
-
Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future.Viruses. 2012 Oct 29;4(11):2650-69. doi: 10.3390/v4112650. Viruses. 2012. PMID: 23202498 Free PMC article. Review.
Cited by
-
Molecular and cellular insights into T cell exhaustion.Nat Rev Immunol. 2015 Aug;15(8):486-99. doi: 10.1038/nri3862. Nat Rev Immunol. 2015. PMID: 26205583 Free PMC article. Review.
-
EOMES is essential for antitumor activity of CD8+ T cells in chronic lymphocytic leukemia.Leukemia. 2021 Nov;35(11):3152-3162. doi: 10.1038/s41375-021-01198-1. Epub 2021 Mar 17. Leukemia. 2021. PMID: 33731848 Free PMC article.
-
Population dynamics and gene regulation of T cells in response to chronic antigen stimulation.Int Immunol. 2023 Feb 11;35(2):67-77. doi: 10.1093/intimm/dxac050. Int Immunol. 2023. PMID: 36334059 Free PMC article. Review.
-
Metabolic barriers to cancer immunotherapy.Nat Rev Immunol. 2021 Dec;21(12):785-797. doi: 10.1038/s41577-021-00541-y. Epub 2021 Apr 29. Nat Rev Immunol. 2021. PMID: 33927375 Free PMC article. Review.
-
Enhancing immune responses to limit chronic immune activation during SIV.J Clin Invest. 2012 May;122(5):1611-4. doi: 10.1172/JCI63389. Epub 2012 Apr 23. J Clin Invest. 2012. PMID: 22523060 Free PMC article.
References
-
- Zinkernagel RM, Doherty PC. Restriction of in vitroT cell mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. - PubMed
-
- Townsend A, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. - PubMed
-
- Morris AG, Lin YL, Askonas BA. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982;295:150–152. - PubMed
-
- Kündig TM, Hengartner H, Zinkernagel RM. T cell dependent interferon gamma exerts an antiviral effect in the central nervous system but not in peripheral sold organs. J Immunol. 1993;150:2316–2321. - PubMed
-
- Ruby J, Ramshaw I. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 1992;10:353–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

