Autologously up-regulated Fc receptor expression and action in airway smooth muscle mediates its altered responsiveness in the atopic asthmatic sensitized state
- PMID: 9560263
- PMCID: PMC20248
- DOI: 10.1073/pnas.95.9.5257
Autologously up-regulated Fc receptor expression and action in airway smooth muscle mediates its altered responsiveness in the atopic asthmatic sensitized state
Abstract
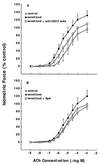
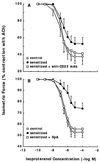
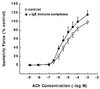
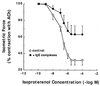
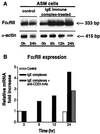
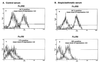
To elucidate the role of IgE-dependent mechanisms in inducing altered airway responsiveness in the atopic asthmatic state, the expression and actions of Fc receptor activation were examined in isolated rabbit tracheal smooth muscle (TSM) tissue and cultured cells passively sensitized with sera from atopic asthmatic patients or nonatopic/nonasthmatic (control) subjects. Relative to control tissues, the atopic asthmatic-sensitized TSM exhibited significantly increased maximal isometric contractility to acetylcholine (P < 0. 01) and attenuated maximal relaxation responses and sensitivity (i.e.,-log ED50) to isoproterenol (P < 0.005). These changes in agonist responsiveness in atopic sensitized TSM were ablated by pretreating the tissues with a blocking mAb to the low affinity receptor for IgE, FcepsilonRII (i.e., CD23) or by depleting the sensitizing serum of its immune complexes. Moreover, in complimentary experiments, exogenous administration of IgE immune complexes to naive TSM produced changes in agonist responsiveness that were qualitatively similar to those obtained in the atopic asthmatic-sensitized state. Extended studies further demonstrated that, in contrast to their respective controls, atopic asthmatic serum-sensitized human and rabbit TSM tissue and cultured cells exhibited markedly induced mRNA and cell surface expression of FcepsilonRII, whereas constitutive expression of the IgG receptor subtype, FcgammaRIII, was unaltered. Finally, the up-regulated mRNA expression of FcepsilonRII observed following exposure of TSM to atopic asthmatic serum or to exogenously administered IgE immune complexes was significantly inhibited by pretreating the tissues or cells with anti-CD23 mAb. Collectively, these observations provide evidence demonstrating that the altered agonist responsiveness in atopic asthmatic sensitized airway smooth muscle is largely attributed to IgE-mediated induction of the autologous expression and activation of FcepsilonRII receptors in the airway smooth muscle itself.
Figures
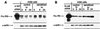
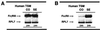

Similar articles
-
Autocrine role of interleukin 1beta in altered responsiveness of atopic asthmatic sensitized airway smooth muscle.J Clin Invest. 1997 Jan 1;99(1):117-24. doi: 10.1172/JCI119122. J Clin Invest. 1997. PMID: 9011565 Free PMC article.
-
Intrinsic ICAM-1/LFA-1 activation mediates altered responsiveness of atopic asthmatic airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2000 Jun;278(6):L1154-63. doi: 10.1152/ajplung.2000.278.6.L1154. Am J Physiol Lung Cell Mol Physiol. 2000. PMID: 10835320
-
Altered expression and action of the low-affinity IgE receptor FcepsilonRII (CD23) in asthmatic airway smooth muscle.J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):575-84. doi: 10.1016/s0091-6749(99)70326-x. J Allergy Clin Immunol. 1999. PMID: 10482830
-
Autocrine regulation of airway smooth muscle responsiveness.Respir Physiol Neurobiol. 2003 Sep 16;137(2-3):263-76. doi: 10.1016/s1569-9048(03)00152-6. Respir Physiol Neurobiol. 2003. PMID: 14516731 Review.
-
The high affinity IgE receptor (FcεRI) expression and function in airway smooth muscle.Pulm Pharmacol Ther. 2013 Feb;26(1):86-94. doi: 10.1016/j.pupt.2012.04.004. Epub 2012 May 8. Pulm Pharmacol Ther. 2013. PMID: 22580035 Review.
Cited by
-
Functional expression of IgG-Fc receptors in human airway smooth muscle cells.Am J Respir Cell Mol Biol. 2011 May;44(5):665-72. doi: 10.1165/rcmb.2009-0371OC. Epub 2010 Jul 1. Am J Respir Cell Mol Biol. 2011. PMID: 20595464 Free PMC article.
-
Endothelin-1 increases cholinergic nerve-mediated contraction of human bronchi via tachykinin synthesis induction.Br J Pharmacol. 2001 Dec;134(7):1447-54. doi: 10.1038/sj.bjp.0704395. Br J Pharmacol. 2001. PMID: 11724750 Free PMC article.
-
CC-chemokine CCL15 expression and possible implications for the pathogenesis of IgE-related severe asthma.Mediators Inflamm. 2012;2012:475253. doi: 10.1155/2012/475253. Epub 2012 Oct 31. Mediators Inflamm. 2012. PMID: 23258953 Free PMC article. Review.
-
Is there a regulatory role of immunoglobulins on tissue forming cells relevant in chronic inflammatory lung diseases?J Allergy (Cairo). 2011;2011:721517. doi: 10.1155/2011/721517. Epub 2011 Nov 2. J Allergy (Cairo). 2011. PMID: 22121383 Free PMC article.
-
G Protein βγ-subunit signaling mediates airway hyperresponsiveness and inflammation in allergic asthma.PLoS One. 2012;7(2):e32078. doi: 10.1371/journal.pone.0032078. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384144 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

