Two types of virus-related particles are found during transmissible gastroenteritis virus morphogenesis
- PMID: 9557690
- PMCID: PMC109630
- DOI: 10.1128/JVI.72.5.4022-4031.1998
Two types of virus-related particles are found during transmissible gastroenteritis virus morphogenesis
Abstract
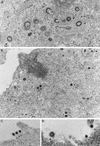
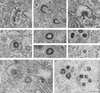
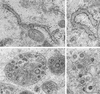
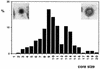
The intracellular assembly of the transmissible gastroenteritis coronavirus (TGEV) was studied in infected swine testis (ST) cells at different postinfection times by using ultrathin sections of conventionally embedded infected cells, freeze-substitution, and methods for detecting viral proteins and RNA at the electron microscopy level. This ultrastructural analysis was focused on the identification of the different viral components that assemble in infected cells, in particular the spherical, potentially icosahedral internal core, a new structural element of the extracellular infectious coronavirus recently characterized by our group. Typical budding profiles and two types of virion-related particles were detected in TGEV-infected cells. While large virions with an electron-dense internal periphery and a clear central area are abundant at perinuclear regions, smaller viral particles, with the characteristic morphology of extracellular virions (exhibiting compact internal cores with polygonal contours) accumulate inside secretory vesicles that reach the plasma membrane. The two types of virions coexist in the Golgi complex of infected ST cells. In nocodazole-treated infected cells, the two types of virions coexist in altered Golgi stacks, while the large secretory vesicles filled with virions found in normal infections are not detected in this case. Treatment of infected cells with the Golgi complex-disrupting agent brefeldin A induced the accumulation of large virions in the cisternae that form by fusion of different membranous compartments. These data, together with the distribution of both types of virions in different cellular compartments, strongly suggest that the large virions are the precursors of the small viral particles and that their transport through a functional Golgi complex is necessary for viral maturation.
Figures
Similar articles
-
Structure and intracellular assembly of the transmissible gastroenteritis coronavirus.Adv Exp Med Biol. 1998;440:341-6. doi: 10.1007/978-1-4615-5331-1_44. Adv Exp Med Biol. 1998. PMID: 9782301
-
Structural maturation of the transmissible gastroenteritis coronavirus.J Virol. 1999 Oct;73(10):7952-64. doi: 10.1128/JVI.73.10.7952-7964.1999. J Virol. 1999. PMID: 10482542 Free PMC article.
-
Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding.J Virol. 1994 Oct;68(10):6523-34. doi: 10.1128/JVI.68.10.6523-6534.1994. J Virol. 1994. PMID: 8083990 Free PMC article.
-
An overview of immunological and genetic methods for detecting swine coronaviruses, transmissible gastroenteritis virus, and porcine respiratory coronavirus in tissues.Adv Exp Med Biol. 1997;412:37-46. doi: 10.1007/978-1-4899-1828-4_4. Adv Exp Med Biol. 1997. PMID: 9191988 Review.
-
Budding events in herpesvirus morphogenesis.Virus Res. 2004 Dec;106(2):167-80. doi: 10.1016/j.virusres.2004.08.013. Virus Res. 2004. PMID: 15567495 Review.
Cited by
-
Transmissible Gastroenteritis Virus: An Update Review and Perspective.Viruses. 2023 Jan 27;15(2):359. doi: 10.3390/v15020359. Viruses. 2023. PMID: 36851573 Free PMC article. Review.
-
Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space.Cells. 2021 Feb 26;10(3):503. doi: 10.3390/cells10030503. Cells. 2021. PMID: 33652973 Free PMC article. Review.
-
EIF4A2 interacts with the membrane protein of transmissible gastroenteritis coronavirus and plays a role in virus replication.Res Vet Sci. 2019 Apr;123:39-46. doi: 10.1016/j.rvsc.2018.12.005. Epub 2018 Dec 17. Res Vet Sci. 2019. PMID: 30583231 Free PMC article.
-
Electron microscopy studies of the coronavirus ribonucleoprotein complex.Protein Cell. 2017 Mar;8(3):219-224. doi: 10.1007/s13238-016-0352-8. Protein Cell. 2017. PMID: 28044277 Free PMC article. No abstract available.
-
Tubulins interact with porcine and human S proteins of the genus Alphacoronavirus and support successful assembly and release of infectious viral particles.Virology. 2016 Oct;497:185-197. doi: 10.1016/j.virol.2016.07.022. Epub 2016 Jul 30. Virology. 2016. PMID: 27479465 Free PMC article.
References
-
- Arnheiter H, Dubois-Dalcq M, Lazzarini R A. Direct visualization of protein transport and processing in the living cell by microinjection of specific antibodies. Cell. 1984;39:99–109. - PubMed
-
- Bendayan M. Ultrastructural localization of nucleic acids by the use of enzyme-gold complexes. J Histochem Cytochem. 1981;29:531–541. - PubMed
-
- Bendayan M. The enzyme-gold cytochemical approach: a review. In: Hayat M A, editor. Colloidal gold: principles, methods, and applications. Vol. 2. San Diego, Calif: Academic Press, Inc.; 1989. pp. 117–147.
-
- Booy F P. Cryoelectron microscopy. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 21–54.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

