Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins
- PMID: 9557669
- PMCID: PMC109609
- DOI: 10.1128/JVI.72.5.3851-3858.1998
Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins
Abstract
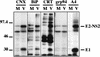
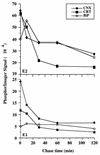
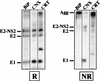
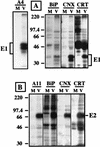
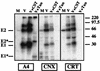
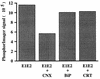
The hepatitis C virus (HCV) genome encodes two envelope glycoproteins (E1 and E2) which interact noncovalently to form a heterodimer (E1-E2). During the folding and assembly of HCV glycoproteins, a large portion of these proteins are trapped in aggregates, reducing the efficiency of native E1-E2 complex assembly. To better understand this phenomenon and to try to increase the efficiency of HCV glycoprotein folding, endoplasmic reticulum chaperones potentially interacting with these proteins were studied. Calnexin, calreticulin, and BiP were shown to interact with E1 and E2, whereas no interaction was detected between GRP94 and HCV glycoproteins. The association of HCV glycoproteins with calnexin and calreticulin was faster than with BiP, and the kinetics of interaction with calnexin and calreticulin were very similar. However, calreticulin and BiP interacted preferentially with aggregates whereas calnexin preferentially associated with monomeric forms of HCV glycoproteins or noncovalent complexes. Tunicamycin treatment inhibited the binding of HCV glycoproteins to calnexin and calreticulin, indicating the importance of N-linked oligosaccharides for these interactions. The effect of the co-overexpression of each chaperone on the folding of HCV glycoproteins was also analyzed. However, the levels of native E1-E2 complexes were not increased. Together, our data suggest that calnexin plays a role in the productive folding of HCV glycoproteins whereas calreticulin and BiP are probably involved in a nonproductive pathway of folding.
Figures
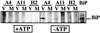


Similar articles
-
Rubella virus glycoprotein interaction with the endoplasmic reticulum calreticulin and calnexin.Arch Virol. 2001;146(1):1-14. doi: 10.1007/s007050170186. Arch Virol. 2001. PMID: 11266204
-
[The role of chaperone proteins in the assembly of envelope proteins of hepatitis C virus].Bull Mem Acad R Med Belg. 1998;153(7-9):343-9; discussion 350-1. Bull Mem Acad R Med Belg. 1998. PMID: 10100398 French.
-
Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin.J Virol. 1996 Feb;70(2):778-86. doi: 10.1128/JVI.70.2.778-786.1996. J Virol. 1996. PMID: 8551615 Free PMC article.
-
Calnexin, calreticulin, and Bip/Kar2p in protein folding.Cold Spring Harb Symp Quant Biol. 1995;60:405-15. doi: 10.1101/sqb.1995.060.01.045. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824414 Review. No abstract available.
-
Biosynthesis, assembly and secretion of coagulation factor VIII.Blood Coagul Fibrinolysis. 1997 Dec;8 Suppl 2:S3-14. Blood Coagul Fibrinolysis. 1997. PMID: 9607108 Review.
Cited by
-
Virus-induced ER stress and the unfolded protein response.Front Plant Sci. 2012 Dec 28;3:293. doi: 10.3389/fpls.2012.00293. eCollection 2012. Front Plant Sci. 2012. PMID: 23293645 Free PMC article.
-
GRP78 targeting: Hitting two birds with a stone.Life Sci. 2020 Nov 1;260:118317. doi: 10.1016/j.lfs.2020.118317. Epub 2020 Aug 22. Life Sci. 2020. PMID: 32841659 Free PMC article. Review.
-
Iminosugars: A host-targeted approach to combat Flaviviridae infections.Antiviral Res. 2020 Dec;184:104881. doi: 10.1016/j.antiviral.2020.104881. Epub 2020 Aug 5. Antiviral Res. 2020. PMID: 32768411 Free PMC article. Review.
-
Landscape of protein-protein interactions during hepatitis C virus assembly and release.Microbiol Spectr. 2024 Feb 6;12(2):e0256222. doi: 10.1128/spectrum.02562-22. Epub 2024 Jan 17. Microbiol Spectr. 2024. PMID: 38230952 Free PMC article. Review.
-
Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein.J Virol. 2005 Sep;79(17):11095-104. doi: 10.1128/JVI.79.17.11095-11104.2005. J Virol. 2005. PMID: 16103160 Free PMC article.
References
-
- Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–129. - PubMed
-
- Cannon K S, Hebert D N, Helenius A. Glycan-dependent and -independent association of vesicular stomatitis virus G protein with calnexin. J Biol Chem. 1996;271:14280–14284. - PubMed
-
- Chamberlain J P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979;98:132–135. - PubMed
-
- Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

