Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes
- PMID: 9529087
- PMCID: PMC108094
- DOI: 10.1128/IAI.66.4.1601-1606.1998
Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes
Abstract
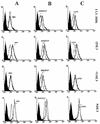
In Plasmodium falciparum malaria, large proportions of resident macrophages and circulating monocytes and leukocytes contain massive amounts of the malarial pigment, hemozoin. Previous studies have shown that important functions (e.g., the generation of the oxidative burst, the ability to repeat phagocytosis, and protein kinase C activity) were severely impaired in hemozoin-loaded monocytes. Expression of membrane antigens directly involved in the immune response and in the phagocytic process, and/or under protein kinase C control, in hemozoin-loaded human monocytes was studied. Expression of major histocompatibility complex (MHC) class II after gamma interferon stimulation was blocked in hemozoin-loaded monocytes at the protein expression and gene transcription levels but was preserved in control monocytes loaded with opsonized latex beads or anti-D(Rho)-immunoglobulin G (IgG)-opsonized human erythrocytes. Expression of CD54 (intracellular adhesion molecule 1) and CD11c (p150,95 integrin) was also decreased in hemozoin-loaded monocytes. Expression of MHC class I, CD16 (low-affinity Fc receptor for aggregated IgG), CD32 (low-affinity Fc receptor for aggregated IgG), CD64 (high-affinity receptor for IgG), CD11b (receptor for complement component iC3b [CR3]), CD35 (receptor for complement components C3b and C4b [CR1]), and CD36 (non-class-A scavenger receptor) was not specifically affected by hemozoin loading. These results suggest that hemozoin loading may contribute to the impairment of the immune response and the derangement of antigen presentation reported in previous studies of P. falciparum malaria.
Figures
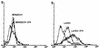


Similar articles
-
Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect.J Immunol. 2004 Sep 15;173(6):4066-74. doi: 10.4049/jimmunol.173.6.4066. J Immunol. 2004. PMID: 15356156
-
Neutrophils and monocytes from subjects with the Mediterranean G6PD variant: effect of Plasmodium falciparum hemozoin on G6PD activity, oxidative burst and cytokine production.Eur Cytokine Netw. 1998 Sep;9(3):239-45. Eur Cytokine Netw. 1998. PMID: 9831172
-
IL-10 augments CD23 expression on U937 cells and down-regulates IL-4-driven CD23 expression on cultured human blood monocytes: effects of IL-10 and other cytokines on cell phenotype and phagocytosis.Immunology. 1995 Jun;85(2):311-7. Immunology. 1995. PMID: 7642222 Free PMC article.
-
Hemozoin and the human monocyte--a brief review of their interactions.Parassitologia. 2008 Jun;50(1-2):143-5. Parassitologia. 2008. PMID: 18693582 Review.
-
Malarial hemozoin: from target to tool.Biochim Biophys Acta. 2014 Jun;1840(6):2032-41. doi: 10.1016/j.bbagen.2014.02.009. Epub 2014 Feb 17. Biochim Biophys Acta. 2014. PMID: 24556123 Free PMC article. Review.
Cited by
-
Involvement of inflammatory chemokines in survival of human monocytes fed with malarial pigment.Infect Immun. 2010 Nov;78(11):4912-21. doi: 10.1128/IAI.00455-10. Epub 2010 Aug 23. Infect Immun. 2010. PMID: 20732999 Free PMC article.
-
Comparative analysis of gene expression changes mediated by individual constituents of hemozoin.Chem Res Toxicol. 2009 Mar 16;22(3):433-45. doi: 10.1021/tx8002752. Chem Res Toxicol. 2009. PMID: 19191707 Free PMC article.
-
Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells.PLoS Pathog. 2007 Oct 12;3(10):1380-7. doi: 10.1371/journal.ppat.0030143. PLoS Pathog. 2007. PMID: 17937497 Free PMC article.
-
The Case for the Use of PPARγ Agonists as an Adjunctive Therapy for Cerebral Malaria.PPAR Res. 2012;2012:513865. doi: 10.1155/2012/513865. Epub 2011 Jun 9. PPAR Res. 2012. PMID: 21772838 Free PMC article.
-
Simple flow cytometric detection of haemozoin containing leukocytes and erythrocytes for research on diagnosis, immunology and drug sensitivity testing.Malar J. 2011 Mar 31;10:74. doi: 10.1186/1475-2875-10-74. Malar J. 2011. PMID: 21453521 Free PMC article.
References
-
- Aikawa M, Suzuki M, Gutierrez Y. Pathology of malaria. In: Kreier J P, editor. Malaria, pathology, vector studies, and culture. Vol. 2. New York, N.Y: Academic Press; 1980. pp. 47–102.
-
- Alessio M, Ghigo D, Garbarino G, Geuna M, Malavasi F. Analysis of the human CD36 leucocyte differentiation antigen by means of the monoclonal antibody NL07. Cell Immuno. 1991;137:487–500. - PubMed
-
- Blackford J, Reid H W, Pappin D J C, Bowers F S, Wilkinson J M. A monoclonal antibody 3/22 to rabbit CD11c which induces homotypic T cell aggregation. Evidence that ICAM-1 is a ligand for CD11c/CD18. Eur J Immunol. 1996;26:525–531. - PubMed
-
- Brasseur P, Agrapart M, Ballet J J, Druilhe P, Warrell M J, Tharavanij S. Impaired cell-mediated immunity in Plasmodium falciparum-infected patients with high parasitemia and cerebral malaria. Clin Immunol Immunopathol. 1983;27:38–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

