Inactivation of p16 in human mammary epithelial cells by CpG island methylation
- PMID: 9528751
- PMCID: PMC121409
- DOI: 10.1128/MCB.18.4.1793
Inactivation of p16 in human mammary epithelial cells by CpG island methylation
Abstract
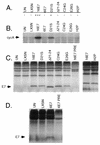
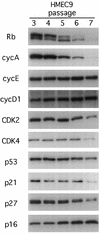
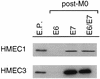
Proliferation of human mammary epithelial cells (HMEC) is limited to a few passages in culture due to an arrest in G1 termed selection or mortality stage 0, M0. A small number of cells spontaneously escape M0, continue to proliferate in culture, and then enter a second mortality stage, M1, at which they senesce. Evidence that M0 involves the Rb pathway comes from the observation that expression of human papillomavirus type 16 E7 alleviates the M0 proliferation block, and we further show that the Rb-binding region of E7 is required to allow cells to bypass M0. In contrast, E6 does not prevent HMEC from entering M0 but, rather, is involved in M1 bypass. Here we show that inactivation of the D-type cyclin-dependent kinase inhibitor p16INK4A is associated with escape from the M0 proliferation block. Early-passage HMEC express readily detectable amounts of p16 protein, whereas normal or E6-expressing HMEC that escaped M0 expressed markedly reduced amounts of p16 mRNA and protein. This initial reduction of p16 expression was associated with limited methylation of the p16 promoter region CpG island. At later passages, a further reduction in p16 expression occurred, accompanied by increased CpG island methylation. In contrast, reduction of p16 expression did not occur in E7-expressing HMEC that bypassed M0, due to inactivation of Rb. These observations in the E6-expressing HMEC correlate well with the finding that CpG island methylation is a mechanism of p16 inactivation in the development of human tumors, including breast cancer.
Figures
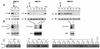
Similar articles
-
Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells.Oncogene. 1996 Apr 18;12(8):1773-9. Oncogene. 1996. PMID: 8622898
-
Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation.Oncogene. 1998 Jul 16;17(2):199-205. doi: 10.1038/sj.onc.1201919. Oncogene. 1998. PMID: 9674704
-
Aberrant de novo methylation of the p16INK4A CpG island is initiated post gene silencing in association with chromatin remodelling and mimics nucleosome positioning.Hum Mol Genet. 2009 Aug 15;18(16):3098-109. doi: 10.1093/hmg/ddp251. Epub 2009 May 28. Hum Mol Genet. 2009. PMID: 19477956
-
Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells.Int J Biochem Cell Biol. 2002 Nov;34(11):1382-94. doi: 10.1016/s1357-2725(02)00047-x. Int J Biochem Cell Biol. 2002. PMID: 12200033 Review.
-
Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells.Leukemia. 1998 Jun;12(6):845-59. doi: 10.1038/sj.leu.2401043. Leukemia. 1998. PMID: 9639410 Review.
Cited by
-
Methylation status of p16 INK4A tumor suppressor gene in Iranian patients with sporadic breast cancer.J Cancer Res Clin Oncol. 2009 Aug;135(8):991-6. doi: 10.1007/s00432-008-0534-8. Epub 2009 Jan 6. J Cancer Res Clin Oncol. 2009. PMID: 19125298
-
p16(Ink4a) interferes with Abelson virus transformation by enhancing apoptosis.J Virol. 2004 Apr;78(7):3304-11. doi: 10.1128/jvi.78.7.3304-3311.2004. J Virol. 2004. PMID: 15016851 Free PMC article.
-
Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway.Mol Biol Cell. 2005 Mar;16(3):1491-9. doi: 10.1091/mbc.e04-07-0652. Epub 2005 Jan 12. Mol Biol Cell. 2005. PMID: 15647378 Free PMC article.
-
Immortalization and transformation of human mammary epithelial cells by a tumor-derived Myc mutant.Breast Cancer Res Treat. 2009 Jul;116(2):281-94. doi: 10.1007/s10549-008-0127-x. Epub 2008 Jul 20. Breast Cancer Res Treat. 2009. PMID: 18642118 Free PMC article.
-
Immortalized cells as experimental models to study cancer.Cytotechnology. 2004 Jun;45(1-2):47-59. doi: 10.1007/s10616-004-5125-1. Cytotechnology. 2004. PMID: 19003243 Free PMC article.
References
-
- Banks L, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
