A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences
- PMID: 9525583
- PMCID: PMC109708
- DOI: 10.1128/JVI.72.4.2663-2670.1998
A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences
Abstract
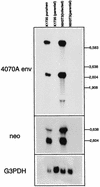
Previously we reported the presence of a replication-competent retrovirus in supernatant from a vector-producing line derived from a widely used split-function amphotropic packaging cell line. Rigorous routine screening of all retroviral stocks produced in our laboratory has not, previously or since, indicated the presence of such a virus. Replication-competent retroviruses have never previously been used in our laboratory, and stringent screening of all routinely used cell lines has not revealed the presence of any helper viruses. Therefore, it is highly unlikely that this virus represents an adventitious cross-contaminant or had been imported unknowingly with our cell line stocks. PCR studies with DNA from infected cell lines and Northern blot analysis and reverse transcriptase PCR with RNA from infected cells suggest that the helper virus arose by recombination events, at sites of partial homology, between sequences in the vector, one of the packaging constructs, and endogenous retroviral elements. These recombinations were not present in stocks of the packaging cell line or in an initial stock of the vector-producing line, indicating that these events occurred while the vector-producing line was being passaged for harvest of supernatant stocks.
Figures




Similar articles
-
Characterization of recombinant helper retroviruses from Moloney-based vectors in ecotropic and amphotropic packaging cell lines.Virology. 1991 Feb;180(2):849-52. doi: 10.1016/0042-6822(91)90105-k. Virology. 1991. PMID: 1989392
-
An array of murine leukemia virus-related elements is transmitted and expressed in a primate recipient of retroviral gene transfer.J Virol. 1996 Feb;70(2):887-97. doi: 10.1128/JVI.70.2.887-897.1996. J Virol. 1996. PMID: 8551628 Free PMC article.
-
Packaging of endogenous retroviral sequences in retroviral vectors produced by murine and human packaging cells.J Virol. 1998 Apr;72(4):2671-6. doi: 10.1128/JVI.72.4.2671-2676.1998. J Virol. 1998. PMID: 9525584 Free PMC article.
-
Safety considerations in vector development.Somat Cell Mol Genet. 2001 Nov;26(1-6):147-58. doi: 10.1023/a:1021082815013. Somat Cell Mol Genet. 2001. PMID: 12465466 Review.
-
Gammaretroviral vectors: biology, technology and application.Viruses. 2011 Jun;3(6):677-713. doi: 10.3390/v3060677. Epub 2011 Jun 3. Viruses. 2011. PMID: 21994751 Free PMC article. Review.
Cited by
-
Design of a trans protease lentiviral packaging system that produces high titer virus.Retrovirology. 2007 Dec 28;4:96. doi: 10.1186/1742-4690-4-96. Retrovirology. 2007. PMID: 18163907 Free PMC article.
-
A reporter system for replication-competent gammaretroviruses: the inGluc-MLV-DERSE assay.Gene Ther. 2013 Feb;20(2):169-76. doi: 10.1038/gt.2012.18. Epub 2012 Mar 8. Gene Ther. 2013. PMID: 22402321 Free PMC article.
-
Expression Analysis of GDNF/RET Signaling Pathway in Human AD-MSCs Grown in HEK 293 Conditioned Medium (HEK293-CM).Cell Biochem Biophys. 2020 Dec;78(4):531-539. doi: 10.1007/s12013-020-00936-z. Epub 2020 Aug 14. Cell Biochem Biophys. 2020. PMID: 32803668
-
Retroviral Vector Biosafety: Lessons from Sheep.J Biomed Biotechnol. 2003;2003(1):9-12. doi: 10.1155/S1110724303209128. J Biomed Biotechnol. 2003. PMID: 12686718 Free PMC article.
-
Meeting FDA Guidance recommendations for replication-competent virus and insertional oncogenesis testing.Mol Ther Methods Clin Dev. 2022 Dec 2;28:28-39. doi: 10.1016/j.omtm.2022.11.009. eCollection 2023 Mar 9. Mol Ther Methods Clin Dev. 2022. PMID: 36588821 Free PMC article. Review.
References
-
- Allan D, De Koven A, Wild A, Kamel-Reid S, Dube I. Endogenous murine leukemia virus DNA sequences in murine cell lines: implications for gene therapy safety testing by PCR. Leuk Lymphoma. 1996;23:375–381. - PubMed
-
- Anderson W F, McGarrity G J, Moen R C. Report to the NIH recombinant DNA advisory committee on murine replication-competent retrovirus (RCR) assays. Hum Gene Ther. 1993;4:311–321. - PubMed
-
- Chong H, Vile R G. Replication-competent retrovirus produced by a ‘split-function’ third generation amphotropic packaging cell line. Gene Ther. 1996;3:624–629. - PubMed
-
- Cornetta K, Morgan R A, Anderson W F. Safety issues related to retroviral-mediated gene transfer in humans. Hum Gene Ther. 1991;2:5–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

