Identification of a transient excision intermediate at the crossroads between DNA polymerase extension and proofreading pathways
- PMID: 9520396
- PMCID: PMC19866
- DOI: 10.1073/pnas.95.7.3507
Identification of a transient excision intermediate at the crossroads between DNA polymerase extension and proofreading pathways
Abstract
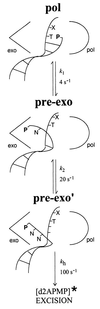
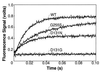
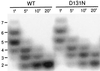
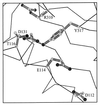
DNA polymerases achieve accurate DNA replication through a delicate balance between primer elongation and proofreading. While insufficient proofreading results in DNA replication errors, indiscriminate removal of correct along with incorrect nucleotides is wasteful and may prevent completion of DNA synthesis. The transition between polymerization and proofreading modes is proposed to be governed by a kinetic barrier that prevents proofreading unless the rate of primer elongation is significantly reduced by the presence of an incorrect base pair at the primer-terminus. We have used mutational analysis, coupled with a sensitive, fluorescence-based assay to characterize intermediate steps in the proofreading pathway. A highly fluorescent complex forms between the bacteriophage T4 DNA polymerase and DNA primer-templates labeled at the 3' terminus with the base analog 2-aminopurine. Formation of the fluorescent complex appears to be a rate-determining step in the proofreading pathway and is impaired for several mutator T4 DNA polymerases with amino acid substitutions in the exonuclease domain. Although these mutant DNA polymerases are proficient in hydrolysis, their reduced ability to form the fluorescent complex imposes a higher kinetic barrier. As a consequence, the mutant DNA polymerases proofread less frequently, resulting in more DNA replication errors.
Figures
Similar articles
-
The proofreading pathway of bacteriophage T4 DNA polymerase.J Biol Chem. 1998 Sep 4;273(36):22969-76. doi: 10.1074/jbc.273.36.22969. J Biol Chem. 1998. PMID: 9722519
-
Motif A of bacteriophage T4 DNA polymerase: role in primer extension and DNA replication fidelity. Isolation of new antimutator and mutator DNA polymerases.J Biol Chem. 1994 Feb 25;269(8):5635-43. J Biol Chem. 1994. PMID: 8119900
-
DNA polymerase mutagenic bypass and proofreading of endogenous DNA lesions.Mutat Res. 1999 Mar 8;424(1-2):221-36. doi: 10.1016/s0027-5107(99)00021-4. Mutat Res. 1999. PMID: 10064863
-
Learning about DNA polymerase function by studying antimutator DNA polymerases.Trends Biochem Sci. 1995 Apr;20(4):136-40. doi: 10.1016/s0968-0004(00)88987-2. Trends Biochem Sci. 1995. PMID: 7770910 Review.
-
Antimutator variants of DNA polymerases.Crit Rev Biochem Mol Biol. 2011 Dec;46(6):548-70. doi: 10.3109/10409238.2011.620941. Epub 2011 Oct 6. Crit Rev Biochem Mol Biol. 2011. PMID: 21977975 Free PMC article. Review.
Cited by
-
Characterization of photophysical and base-mimicking properties of a novel fluorescent adenine analogue in DNA.Nucleic Acids Res. 2011 May;39(10):4513-24. doi: 10.1093/nar/gkr010. Epub 2011 Jan 29. Nucleic Acids Res. 2011. PMID: 21278417 Free PMC article.
-
Single-molecule measurements of synthesis by DNA polymerase with base-pair resolution.Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21109-14. doi: 10.1073/pnas.0908640106. Epub 2009 Dec 2. Proc Natl Acad Sci U S A. 2009. PMID: 19955412 Free PMC article.
-
Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice.J Virol. 2006 Oct;80(19):9391-401. doi: 10.1128/JVI.00605-06. J Virol. 2006. PMID: 16973545 Free PMC article.
-
Identification of a mutant DNA polymerase delta in Saccharomyces cerevisiae with an antimutator phenotype for frameshift mutations.Genetics. 2001 May;158(1):177-86. doi: 10.1093/genetics/158.1.177. Genetics. 2001. PMID: 11333228 Free PMC article.
-
Direct observation of translocation in individual DNA polymerase complexes.J Biol Chem. 2012 Apr 13;287(16):13407-21. doi: 10.1074/jbc.M111.338418. Epub 2012 Feb 29. J Biol Chem. 2012. PMID: 22378784 Free PMC article.
References
-
- Johnson K A. Annu Rev Biochem. 1993;62:685–713. - PubMed
-
- Goodman M F, Creighton S, Bloom L B, Petruska J. Crit Rev Biochem Mol Biol. 1993;28:83–126. - PubMed
-
- Cowart M C, Gibson K J, Allen D J, Benkovic S J. Biochemistry. 1989;28:1975–1983. - PubMed
-
- Marquez L A, Reha-Krantz L J. J Biol Chem. 1996;271:28903–28911. - PubMed
-
- Ollis D L, Brick P, Hamlin R, Xuong N G, Steitz T A. Nature (London) 1985;313:762–766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

