When an ATPase is not an ATPase: at low temperatures the C-terminal domain of the ABC transporter CvaB is a GTPase
- PMID: 9515899
- PMCID: PMC107029
- DOI: 10.1128/JB.180.6.1347-1353.1998
When an ATPase is not an ATPase: at low temperatures the C-terminal domain of the ABC transporter CvaB is a GTPase
Abstract
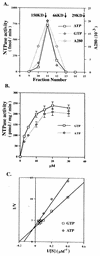
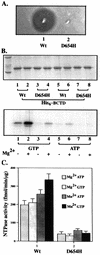
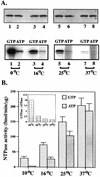
The ATP-binding cassette (ABC) transporters belong to a large superfamily of proteins which share a common function and a common nucleotide-binding domain. The CvaB protein from Escherichia coli is a member of the bacterial ABC exporter subfamily and is essential for the export of the peptide antibiotic colicin V. Here we report that, surprisingly, the CvaB carboxyl-terminal nucleotide-binding domain (BCTD) can be preferentially cross-linked to GTP but not to ATP at low temperatures. The cross-linking is Mg2+ and Mn2+ dependent. However, BCTD possesses similar GTPase and ATPase activities at 37 degrees C, with the same kinetic parameters and with similar responses to inhibitors. Moreover, a point mutation (D654H) in CvaB that completely abolishes colicin V secretion severely impairs both GTPase and ATPase activities in the corresponding BCTD, indicating that the two activities are from the same enzyme. Interestingly, hydrolysis activity of ATP is much more cold sensitive than that of GTP: BCTD possesses mainly GTP hydrolysis activity at 10 degrees C, consistent with the cross-linking results. These findings suggest a novel mechanism for an ABC protein-mediated transport with specificity for GTP hydrolysis.
Figures

Similar articles
-
Molecular basis for differential nucleotide binding of the nucleotide-binding domain of ABC-transporter CvaB.Biochemistry. 2006 Dec 5;45(48):14473-80. doi: 10.1021/bi061506i. Biochemistry. 2006. PMID: 17128986 Free PMC article.
-
Processing of colicin V-1, a secretable marker protein of a bacterial ATP binding cassette export system, requires membrane integrity, energy, and cytosolic factors.J Biol Chem. 1996 Nov 8;271(45):28057-63. doi: 10.1074/jbc.271.45.28057. J Biol Chem. 1996. PMID: 8910417
-
Nucleotide-dependent dimerization of the C-terminal domain of the ABC transporter CvaB in colicin V secretion.J Bacteriol. 2006 Apr;188(7):2383-91. doi: 10.1128/JB.188.7.2383-2391.2006. J Bacteriol. 2006. PMID: 16547024 Free PMC article.
-
The core domain of the tissue transglutaminase Gh hydrolyzes GTP and ATP.Biochemistry. 1997 Sep 30;36(39):11655-64. doi: 10.1021/bi970545e. Biochemistry. 1997. PMID: 9305955
-
The CydDC family of transporters.Res Microbiol. 2019 Nov-Dec;170(8):407-416. doi: 10.1016/j.resmic.2019.06.003. Epub 2019 Jul 3. Res Microbiol. 2019. PMID: 31279084 Review.
Cited by
-
Streptococcus suis MsmK: Novel Cell Division Protein Interacting with FtsZ and Maintaining Cell Shape.mSphere. 2021 Mar 17;6(2):e00119-21. doi: 10.1128/mSphere.00119-21. mSphere. 2021. PMID: 33731468 Free PMC article.
-
Mutational analysis of Cvab, an ABC transporter involved in the secretion of active colicin V.PLoS One. 2012;7(4):e35382. doi: 10.1371/journal.pone.0035382. Epub 2012 Apr 23. PLoS One. 2012. PMID: 22539970 Free PMC article.
-
Molecular basis for differential nucleotide binding of the nucleotide-binding domain of ABC-transporter CvaB.Biochemistry. 2006 Dec 5;45(48):14473-80. doi: 10.1021/bi061506i. Biochemistry. 2006. PMID: 17128986 Free PMC article.
-
Antibiotic resistance ABCF proteins reset the peptidyl transferase centre of the ribosome to counter translational arrest.Nucleic Acids Res. 2018 Apr 20;46(7):3753-3763. doi: 10.1093/nar/gky050. Nucleic Acids Res. 2018. PMID: 29415157 Free PMC article.
-
Conformational changes in a multidrug resistance ABC transporter DrrAB: Fluorescence-based approaches to study substrate binding.Arch Biochem Biophys. 2018 Nov 15;658:31-45. doi: 10.1016/j.abb.2018.09.017. Epub 2018 Sep 20. Arch Biochem Biophys. 2018. PMID: 30243711 Free PMC article.
References
-
- Al-Shawi M K, Senior A E. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J Biol Chem. 1993;268:4197–4206. - PubMed
-
- Ames G F-L, Nikaido K, Groarke J, Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. J Biol Chem. 1989;264:3998–4002. - PubMed
-
- Ames G F-L, Mimura C S, Holbrook S R, Shyamala V. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv Enzymol. 1992;65:1–47. - PubMed
-
- Anderson M P, Berger H A, Rich D P, Gregory R J, Smith A E, Welsh M J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. - PubMed
-
- Anderson M P, Welsh M J. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

