A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2
- PMID: 9499075
- PMCID: PMC109514
- DOI: 10.1128/JVI.72.3.2183-2191.1998
A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2
Abstract
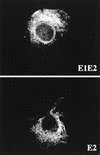
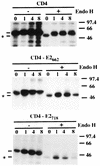
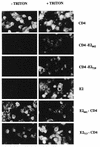
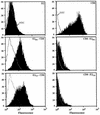
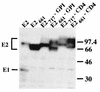
The hepatitis C virus (HCV) genome encodes two envelope glycoproteins (E1 and E2). These glycoproteins interact to formin a noncovalent heterodimeric complex which is retained in the endoplasmic reticulum (ER). To identify whether E1 and/or E2 contains an ER-targeting signal potentially involved in ER retention of the E1-E2 complex, these proteins were expressed alone and their intracellular localization was studied. Due to misfolding of E1 in the absence of E2, no conclusion on the localization of its native form could be drawn from the expression of E1 alone. E2 expressed in the absence of E1 was shown to be retained in the ER similarly to E1-E2 complex. Chimeric proteins in which E2 domains were exchanged with corresponding domains of a protein normally transported to the plasma membrane (CD4) were constructed to identify the sequence responsible for its ER retention. The transmembrane domain (TMD) of E2 (C-terminal 29 amino acids) was shown to be sufficient for retention of the ectodomain of CD4 in the ER compartment. Replacement of the E2 TMD by the anchor signal of CD4 or a glycosyl phosphatidylinositol (GPI) moiety led to its expression on the cell surface. In addition, replacement of the E2 TMD by the anchor signal of CD4 or a GPI moiety abolished the formation of E1-E2 complexes. Together, these results suggest that, besides having a role as a membrane anchor, the TMD of E2 is involved in both complex formation and intracellular localization.
Figures


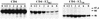
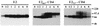


Similar articles
-
The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum.J Virol. 1999 Apr;73(4):2641-9. doi: 10.1128/JVI.73.4.2641-2649.1999. J Virol. 1999. PMID: 10074109 Free PMC article.
-
Characterization of an endoplasmic reticulum retention signal in the rubella virus E1 glycoprotein.J Virol. 1997 Oct;71(10):7670-80. doi: 10.1128/JVI.71.10.7670-7680.1997. J Virol. 1997. PMID: 9311850 Free PMC article.
-
The C-terminal region of the hepatitis C virus E1 glycoprotein confers localization within the endoplasmic reticulum.J Gen Virol. 1999 Aug;80 ( Pt 8):1943-1947. doi: 10.1099/0022-1317-80-8-1943. J Gen Virol. 1999. PMID: 10466789
-
Topology of hepatitis C virus envelope glycoproteins.Rev Med Virol. 2003 Jul-Aug;13(4):233-41. doi: 10.1002/rmv.391. Rev Med Virol. 2003. PMID: 12820185 Review.
-
[Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins].Ann Biol Clin (Paris). 2007 May-Jun;65(3):237-46. Ann Biol Clin (Paris). 2007. PMID: 17502294 Review. French.
Cited by
-
Genetic Diversity Underlying the Envelope Glycoproteins of Hepatitis C Virus: Structural and Functional Consequences and the Implications for Vaccine Design.Viruses. 2015 Jul 17;7(7):3995-4046. doi: 10.3390/v7072809. Viruses. 2015. PMID: 26193307 Free PMC article. Review.
-
Cell fusion activity of hepatitis C virus envelope proteins.J Virol. 2000 Jun;74(11):5066-74. doi: 10.1128/jvi.74.11.5066-5074.2000. J Virol. 2000. PMID: 10799580 Free PMC article.
-
Persistent and transient replication of full-length hepatitis C virus genomes in cell culture.J Virol. 2002 Apr;76(8):4008-21. doi: 10.1128/jvi.76.8.4008-4021.2002. J Virol. 2002. PMID: 11907240 Free PMC article.
-
Identification of a novel drug lead that inhibits HCV infection and cell-to-cell transmission by targeting the HCV E2 glycoprotein.PLoS One. 2014 Oct 30;9(10):e111333. doi: 10.1371/journal.pone.0111333. eCollection 2014. PLoS One. 2014. PMID: 25357246 Free PMC article.
-
Claudin-6 and Occludin Natural Variants Found in a Patient Highly Exposed but Not Infected with Hepatitis C Virus (HCV) Do Not Confer HCV Resistance In Vitro.PLoS One. 2015 Nov 12;10(11):e0142539. doi: 10.1371/journal.pone.0142539. eCollection 2015. PLoS One. 2015. PMID: 26561856 Free PMC article.
References
-
- Ahn K, Szczesna-Skorupa E, Kemper B. The amino-terminal 29 amino acids of cytochrome P450 2C1 are sufficient for retention in the endoplasmic reticulum. J Biol Chem. 1993;268:18726–18733. - PubMed
-
- Armstrong J, Patel S. The Golgi sorting domain of coronavirus E1 protein. J Cell Sci. 1991;98:567–575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

