Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B
- PMID: 9499033
- PMCID: PMC109472
- DOI: 10.1128/JVI.72.3.1826-1833.1998
Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B
Abstract
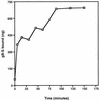
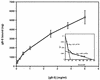
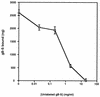
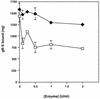
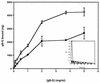
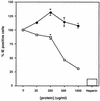
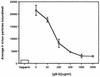
The human cytomegalovirus (HCMV) glycoprotein B (gB) (also known as gpUL55) homolog is an important mediator of virus entry and cell-to-cell dissemination of infection. To examine the potential ligand-binding properties of gB, a soluble form of gB (gB-S) was radiolabeled, purified, and tested in cell-binding experiments. Binding of gB-S to human fibroblast cells was found to occur in a dose-dependent, saturable, and specific manner. Scatchard analysis demonstrated a biphasic plot with the following estimated dissociation constants (Kd): Kd1, 4.96 x 10(-6) M; Kd2, 3.07 x 10(-7) M. Cell surface heparan sulfate proteoglycans (HSPGs) were determined to serve as one class of receptors able to facilitate gB-S binding. Both HSPG-deficient Chinese hamster ovary (CHO) cells and fibroblast cells with enzymatically removed HSPGs had 40% reductions in gB-S binding, whereas removal of chondroitin sulfate had no effect. However, a significant proportion of gB-S was able to associate with the cell surface in the absence of HSPGs via an undefined nonheparin component. Binding affinity analysis of gB-S binding to wild-type CHO-K1 cells demonstrated biphasic binding kinetics (Kd1, 9.85 x 10(-6) M; Kd2, 4.03 x 10(-8) M), whereas gB-S binding to HSPG-deficient CHO-677 cells exhibited single-component binding kinetics (Kd, 7.46 x 10(-6) M). Together, these data suggest that gB-S associates with two classes of cellular receptors. The interaction of gB with its receptors is physiologically relevant, as evidenced by an inhibitory effect on HCMV entry when cells were pretreated with purified gB-S. This inhibition was determined to be manifested at the level of virus attachment. We conclude that gB is a ligand for HCMV that mediates an interaction with a cellular receptor(s) during HCMV infection.
Figures

Similar articles
-
Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans.J Virol. 1997 Jun;71(6):4571-80. doi: 10.1128/JVI.71.6.4571-4580.1997. J Virol. 1997. PMID: 9151851 Free PMC article.
-
Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry.J Virol. 2005 Sep;79(18):11588-97. doi: 10.1128/JVI.79.18.11588-11597.2005. J Virol. 2005. PMID: 16140736 Free PMC article.
-
Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans.J Virol. 2003 Sep;77(18):9922-30. doi: 10.1128/jvi.77.18.9922-9930.2003. J Virol. 2003. PMID: 12941902 Free PMC article.
-
Human cytomegalovirus glycoprotein-receptor interactions.Transplant Proc. 1991 Jun;23(3 Suppl 3):60-3. Transplant Proc. 1991. PMID: 1648838 Review.
-
[The receptor and pathways for human cytomegalovirus entry].Nihon Rinsho. 1998 Jan;56(1):44-9. Nihon Rinsho. 1998. PMID: 9465663 Review. Japanese.
Cited by
-
A peptide inhibitor of cytomegalovirus infection from human hemofiltrate.Antimicrob Agents Chemother. 2013 Oct;57(10):4751-60. doi: 10.1128/AAC.00854-13. Epub 2013 Jul 15. Antimicrob Agents Chemother. 2013. PMID: 23856778 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Human induced pluripotent stem cells are resistant to human cytomegalovirus infection primarily at the attachment level due to the reduced expression of cell-surface heparan sulfate.J Virol. 2024 Mar 19;98(3):e0127823. doi: 10.1128/jvi.01278-23. Epub 2024 Feb 12. J Virol. 2024. PMID: 38345384 Free PMC article.
-
Human cytomegalovirus entry into cells.Curr Opin Virol. 2012 Feb;2(1):37-42. doi: 10.1016/j.coviro.2012.01.001. Epub 2012 Jan 30. Curr Opin Virol. 2012. PMID: 22440964 Free PMC article. Review. No abstract available.
-
Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion.J Virol. 2005 Jun;79(12):7827-37. doi: 10.1128/JVI.79.12.7827-7837.2005. J Virol. 2005. PMID: 15919936 Free PMC article.
References
-
- Adlish J D, Lahijani R S, St. Jeor S C. Identification of a putative cell receptor for human cytomegalovirus. Virology. 1990;176:337–345. - PubMed
-
- Alford C A, Britt W J. Cytomegalovirus. In: Fields B N, Knipe D M, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1981–2010.
-
- Berry N J, Burns D M, Wannamethee G, Grundy J E, Lui S F, Prentice H G, Griffiths P D. Seroepidemiologic studies on the acquisition of antibodies to cytomegalovirus, herpes simplex virus, and human immunodeficiency virus among general hospital patients and those attending a clinic for sexually transmitted diseases. J Med Virol. 1988;24:385–393. - PubMed
-
- Boyle, K. A., and T. Compton. Unpublished results. - PubMed
-
- Britt W J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984;135:369–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

