Regulation of chloride secretion across porcine endometrial epithelial cells by prostaglandin E2
- PMID: 9490813
- PMCID: PMC2230864
- DOI: 10.1111/j.1469-7793.1998.031br.x
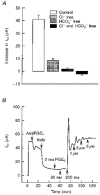
Regulation of chloride secretion across porcine endometrial epithelial cells by prostaglandin E2
Abstract
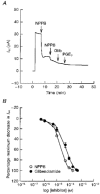
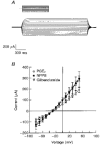
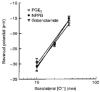
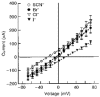
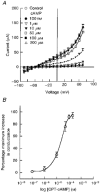
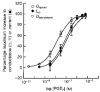
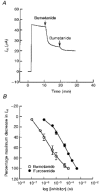
1. The objective of this study was to investigate the mechanism of PGE2 regulation of Cl- transport across glandular endometrial cells grown in primary culture. 2. Most of the basal short circuit current (Isc) was inhibited by luminal addition of 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) or glibenclamide, suggesting the presence of a basally active Cl- conductance in the apical membrane. 3. Basolateral addition of 10 microM PGE2 increased Isc by 41 +/- 3 microA. A similar response was observed when cells were treated with 8-(4-chlorophenylthio) adenosine 3',5'-cyclic monophosphate (CPT-cAMP). Pretreatment of monolayers with NPPB and glibenclamide blocked the PGE2 and cAMP-mediated increase in Isc, suggesting that the effects of PGE2 and cAMP were dependent on the activity of an apical NPPB- and glibenclamide-sensitive conductance. 4. Addition of 50 nM antiPGE2 antibody to the basolateral bathing solution decreased basal Isc by 20 % and shifted the threshold response to exogenous PGE2. This result suggests autocrine regulation of electrogenic Cl- transport by PGE2. 5. Experiments with amphotericin B-permeabilized monolayers revealed that the apical PGE2-activated, NPPB- and glibenclamide-sensitive conductance was Cl- dependent and that the current-voltage relationship and anion permeation properties (SCN->Br- > Cl- > I-) were characteristic of the cystic fibrosis transmembrane conductance regulator (CFTR). 6. Cultured porcine endometrial epithelial cells were specifically labelled with an antibody to a peptide sequence within the regulatory domain of CFTR. 7. The effect of PGE2 was blocked by basolateral addition of bumetanide and furosemide at concentrations that are selective for inhibition of Na+-K+-2Cl-cotransport activity. The effect of bumetanide on Isc was Cl- dependent, suggesting a role for the bumetanide-sensitive transport pathway in Cl- secretion. 8. PGE2 and cAMP also activated an outwardly rectifying basolateral K+ channel which presumably sustains the driving force for electrogenic Cl- efflux across the apical membrane. 9. The concentration-conductance and concentration-Isc response relationships for PGE2 showed that basolateral K+ permeability was rate limiting with respect to transepithelial anion secretion and that activation of a basolateral K+ channel by PGE2 was necessary to achieve maximum rates of Cl- secretion.
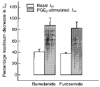
Figures

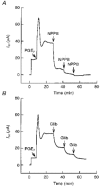

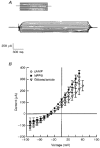

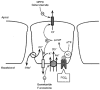
Similar articles
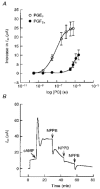
-
Activation of chloride secretion by isoflavone genistein in endometrial epithelial cells.Cell Physiol Biochem. 2013;32(5):1473-86. doi: 10.1159/000356584. Cell Physiol Biochem. 2013. PMID: 24296547
-
Activation of an adenosine 3',5'-cyclic monophosphate-dependent Cl- conductance in response to neurohormonal stimuli in mouse endometrial epithelial cells: the role of cystic fibrosis transmembrane conductance regulator.Biol Reprod. 1999 Feb;60(2):374-80. doi: 10.1095/biolreprod60.2.374. Biol Reprod. 1999. PMID: 9916004
-
Modulation of Cl- secretion by benzimidazolones. II. Coordinate regulation of apical GCl and basolateral GK.Am J Physiol. 1996 Nov;271(5 Pt 1):L785-95. doi: 10.1152/ajplung.1996.271.5.L785. Am J Physiol. 1996. PMID: 8944722
-
The molecular basis of chloride transport in shark rectal gland.J Exp Biol. 1994 Nov;196:405-18. doi: 10.1242/jeb.196.1.405. J Exp Biol. 1994. PMID: 7529818 Review.
-
K+ and Cl- conductances in the distal colon of the rat.Gen Pharmacol. 1998 Sep;31(3):337-42. doi: 10.1016/s0306-3623(97)00458-8. Gen Pharmacol. 1998. PMID: 9703198 Review.
Cited by
-
Trichomonas vaginalis infection impairs anion secretion in vaginal epithelium.PLoS Negl Trop Dis. 2021 Apr 16;15(4):e0009319. doi: 10.1371/journal.pntd.0009319. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33861752 Free PMC article.
-
Advances in Ca2+ modulation of gastrointestinal anion secretion and its dysregulation in digestive disorders (Review).Exp Ther Med. 2020 Nov;20(5):8. doi: 10.3892/etm.2020.9136. Epub 2020 Aug 25. Exp Ther Med. 2020. PMID: 32934673 Free PMC article. Review.
-
Absorptive apical amiloride-sensitive Na+ conductance in human endometrial epithelium.J Physiol. 1998 Dec 1;513 ( Pt 2)(Pt 2):443-52. doi: 10.1111/j.1469-7793.1998.443bb.x. J Physiol. 1998. PMID: 9806994 Free PMC article.
-
Spatial organization of endometrial gene expression at the onset of embryo attachment in pigs.BMC Genomics. 2019 Nov 21;20(1):895. doi: 10.1186/s12864-019-6264-2. BMC Genomics. 2019. PMID: 31752681 Free PMC article.
-
Fluid secretion caused by aerolysin-like hemolysin of Aeromonas sobria in the intestines is due to stimulation of production of prostaglandin E2 via cyclooxygenase 2 by intestinal cells.Infect Immun. 2008 Mar;76(3):1076-82. doi: 10.1128/IAI.01098-07. Epub 2007 Dec 17. Infect Immun. 2008. PMID: 18086811 Free PMC article.
References
-
- Alecozay AA, Harper MJK, Scheken RS, Hanahan DI. Paracrine interactions between platelet-activating factor and prostaglandins in hormonally-treated human luteal phase endometrium in vitro. Journal of Reproduction and Fertility. 1991;91:301–312. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Boldt J, Casas A, Whaley E, Creazzo T, Lewis JB. Potassium dependence for sperm-egg fusion in mice. Journal of Experimental Zoology. 1991;257:245–251. - PubMed
-
- Casslen B, Nilsson B. Human uterine fluid examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin glucose and urea. American Journal of Obstetrics and Gynecology. 1984;150:877–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

