Enteric beta-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection
- PMID: 9488394
- PMCID: PMC108014
- DOI: 10.1128/IAI.66.3.1045-1056.1998
Enteric beta-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection
Erratum in
- Infect Immun 1998 May;66(5):2399
Abstract
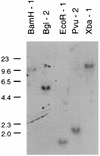
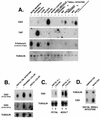
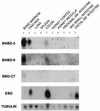
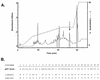
A growing body of evidence suggests that endogenous antibiotics contribute to the innate defense of mammalian mucosal surfaces. In the cow, beta-defensins constitute a large family of antibiotic peptides whose members have been previously isolated from the respiratory and oral mucosa, as well as circulating phagocytic cells. A novel bovine genomic clone with beta-defensin-related sequence [corrected] related to those of these alpha-defensins was isolated and characterized. The corresponding cDNA was isolated from a small intestinal library; its open reading frame predicts a deduced sequence of a novel beta-defensin, which we designate enteric beta-defensin (EBD). Northern blot analysis of a variety of bovine tissues revealed that EBD mRNA is highly expressed in the distal small intestine and colon, anatomic locations distinct from those for previously characterized beta-defensins. EBD mRNA was further localized by in situ hybridization to epithelial cells of the colon and small intestinal crypts. Infection of two calves with the intestinal parasite Cryptosporidium parvum induced 5- and 10-fold increases above control levels of EBD mRNA in intestinal tissues. An anchored-PCR strategy was used to identify other beta-defensin mRNAs expressed in the intestine. In addition to that of EBD, several low-abundance cDNAs which corresponded to other beta-defensin mRNAs were cloned. Most of these clones encoded previously characterized beta-defensins or closely related isoforms, but two encoded a previously uncharacterized prepro-beta-defensin. Northern blot evidence supported that all of these other beta-defensin genes are expressed at levels lower than that of the EBD gene in enteric tissue. Furthermore, some of these beta-defensin mRNAs were abundant in bone marrow, suggesting that in enteric tissue their expression may be in cells of hematopoietic origin. Extracts of small intestinal mucosa obtained from healthy cows have numerous active chromatographic fractions as determined by an antibacterial assay, and one peptide was partially purified. The peptide corresponded to one of the low-abundance cDNAs. This study provides evidence of beta-defensin expression in enteric tissue and that the mRNA encoding a major beta-defensin of enteric tissue, EBD, is inducibly expressed in enteric epithelial cells. These findings support the proposal that beta-defensins may contribute to host defense of enteric mucosa.
Figures



Similar articles
-
Human enteric defensins. Gene structure and developmental expression.J Biol Chem. 1996 Feb 23;271(8):4038-45. doi: 10.1074/jbc.271.8.4038. J Biol Chem. 1996. PMID: 8626737
-
Molecular cloning and characterization of rat genes encoding homologues of human beta-defensins.Infect Immun. 1999 Sep;67(9):4827-33. doi: 10.1128/IAI.67.9.4827-4833.1999. Infect Immun. 1999. PMID: 10456937 Free PMC article.
-
Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel.FEBS Lett. 1993 Jan 4;315(2):187-92. doi: 10.1016/0014-5793(93)81160-2. FEBS Lett. 1993. PMID: 8417977
-
Epithelial antimicrobial peptides: review and significance for oral applications.Crit Rev Oral Biol Med. 1998;9(4):399-414. doi: 10.1177/10454411980090040201. Crit Rev Oral Biol Med. 1998. PMID: 9825219 Review.
-
Alpha-defensins in the gastrointestinal tract.Mol Immunol. 2003 Nov;40(7):463-7. doi: 10.1016/s0161-5890(03)00157-3. Mol Immunol. 2003. PMID: 14568393 Review.
Cited by
-
Induction of cationic chicken liver-expressed antimicrobial peptide 2 in response to Salmonella enterica infection.Infect Immun. 2004 Dec;72(12):6987-93. doi: 10.1128/IAI.72.12.6987-6993.2004. Infect Immun. 2004. PMID: 15557621 Free PMC article.
-
Defensins and innate host defence of the gastrointestinal tract.Gut. 1999 Dec;45(6):911-5. doi: 10.1136/gut.45.6.911. Gut. 1999. PMID: 10562592 Free PMC article. Review. No abstract available.
-
Gallinacin-3, an inducible epithelial beta-defensin in the chicken.Infect Immun. 2001 Apr;69(4):2684-91. doi: 10.1128/IAI.69.4.2684-2691.2001. Infect Immun. 2001. PMID: 11254635 Free PMC article.
-
Paneth cells in farm animals: current status and future direction.J Anim Sci Biotechnol. 2023 Aug 15;14(1):118. doi: 10.1186/s40104-023-00905-5. J Anim Sci Biotechnol. 2023. PMID: 37582766 Free PMC article. Review.
-
The cell biology of cryptosporidium infection.Microbes Infect. 2011 Aug;13(8-9):721-30. doi: 10.1016/j.micinf.2011.03.008. Epub 2011 Mar 31. Microbes Infect. 2011. PMID: 21458585 Free PMC article. Review.
References
-
- Agerberth B, Boman A, Andersson M, Jörnvall H, Mutt V, Boma H G. Isolation of three antibacterial peptides from pigintestine: gastric inhibitory polypeptide(7-42), diazepam-binding inhibitor(32-86) and a novel factor, peptide 3910. Eur J Biochem. 1993;216:623–629. - PubMed
-
- Agerberth B, Lee J-Y, Bergman T, Carlquist M, Boman H G, Mutt V, Jörnvall H. Amino acid sequence of PR-39, isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem. 1991;202:849–854. - PubMed
-
- Beagley K W, Elson C O. Cells and cytokines in mucosal immunity and inflammation. Gastroenterol Clin N Am. 1992;21:347–366. - PubMed
-
- Bensch K W, Raida M, Mägert H-J, Schulz-Knappe P, Forssmann W-G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–335. - PubMed
-
- Bevins C L. Antimicrobial peptides as agents of mucosal immunity. Ciba Found Symp. 1994;186:250–269. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

