CpxP, a stress-combative member of the Cpx regulon
- PMID: 9473036
- PMCID: PMC106961
- DOI: 10.1128/JB.180.4.831-839.1998
CpxP, a stress-combative member of the Cpx regulon
Abstract
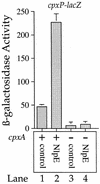
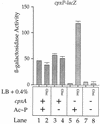
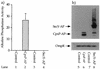
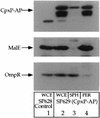
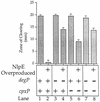
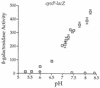
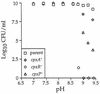
The CpxA/R two-component signal transduction system of Escherichia coli can combat a variety of extracytoplasmic protein-mediated toxicities. The Cpx system performs this function, in part, by increasing the synthesis of the periplasmic protease, DegP. However, other factors are also employed by the Cpx system for this stress-combative function. In an effort to identify these remaining factors, we screened a collection of random lacZ operon fusions for those fusions whose transcription is regulated by CpxA/R. Through this approach, we have identified a new locus, cpxP, whose transcription is stimulated by activation of the Cpx pathway. cpxP specifies a periplasmic protein that can combat the lethal phenotype associated with the synthesis of a toxic envelope protein. In addition, we show that cpxP transcription is strongly induced by alkaline pH in a CpxA-dependent manner and that cpxP and cpx mutant strains display hypersensitivity to growth in alkaline conditions.
Figures



Similar articles
-
Signal detection and target gene induction by the CpxRA two-component system.J Bacteriol. 2003 Apr;185(8):2432-40. doi: 10.1128/JB.185.8.2432-2440.2003. J Bacteriol. 2003. PMID: 12670966 Free PMC article.
-
Tethering of CpxP to the inner membrane prevents spheroplast induction of the cpx envelope stress response.Mol Microbiol. 2000 Sep;37(5):1186-97. doi: 10.1046/j.1365-2958.2000.02074.x. Mol Microbiol. 2000. PMID: 10972835
-
The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli.Genes Dev. 1997 May 1;11(9):1183-93. doi: 10.1101/gad.11.9.1183. Genes Dev. 1997. PMID: 9159399
-
Periplasmic stress and ECF sigma factors.Annu Rev Microbiol. 2001;55:591-624. doi: 10.1146/annurev.micro.55.1.591. Annu Rev Microbiol. 2001. PMID: 11544368 Review.
-
[Cpx two-component regulatory system in gram-negative bacteria--a review].Wei Sheng Wu Xue Bao. 2014 Mar 4;54(3):269-75. Wei Sheng Wu Xue Bao. 2014. PMID: 24984518 Review. Chinese.
Cited by
-
Signature-tagged mutagenesis and co-infection studies demonstrate the importance of P fimbriae in a murine model of urinary tract infection.Pathog Dis. 2015 Jun;73(4):ftv014. doi: 10.1093/femspd/ftv014. Epub 2015 Feb 11. Pathog Dis. 2015. PMID: 25673667 Free PMC article.
-
Genome comparisons provide insights into the role of secondary metabolites in the pathogenic phase of the Photorhabdus life cycle.BMC Genomics. 2016 Aug 3;17:537. doi: 10.1186/s12864-016-2862-4. BMC Genomics. 2016. PMID: 27488257 Free PMC article.
-
Systematic Identification of CpxRA-Regulated Genes and Their Roles in Escherichia coli Stress Response.mSystems. 2022 Oct 26;7(5):e0041922. doi: 10.1128/msystems.00419-22. Epub 2022 Sep 7. mSystems. 2022. PMID: 36069452 Free PMC article.
-
Maintaining Integrity Under Stress: Envelope Stress Response Regulation of Pathogenesis in Gram-Negative Bacteria.Front Cell Infect Microbiol. 2019 Sep 4;9:313. doi: 10.3389/fcimb.2019.00313. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31552196 Free PMC article. Review.
-
Signal detection and target gene induction by the CpxRA two-component system.J Bacteriol. 2003 Apr;185(8):2432-40. doi: 10.1128/JB.185.8.2432-2440.2003. J Bacteriol. 2003. PMID: 12670966 Free PMC article.
References
-
- Albin R, Weber R, Silverman P M. The Cpx proteins of Escherichia coli K12. Immunologic detection of the chromosomal cpxA gene product. J Biol Chem. 1986;261:4698–4705. - PubMed
-
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique EcoRI sites for selection of EcoRI generated recombinant DNA molecules. Gene. 1978;4:121–136. - PubMed
-
- Cosma C L, Danese P N, Carlson J H, Silhavy T J, Snyder W B. Activation of the Cpx two-component signal transduction pathway in Escherichia coli suppresses envelope associated stresses. Mol Microbiol. 1995;18:491–505. - PubMed
-
- Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

