Evaluation of a new competitive immunoassay (BioElisa Syphilis) for screening for Treponema pallidum antibodies at various stages of syphilis
- PMID: 9466741
- PMCID: PMC104542
- DOI: 10.1128/JCM.36.2.358-361.1998
Evaluation of a new competitive immunoassay (BioElisa Syphilis) for screening for Treponema pallidum antibodies at various stages of syphilis
Abstract
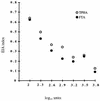
The BioElisa Syphilis, a new competitive enzyme immunoassay (EIA) for Treponema pallidum whole antigen that uses specific human immunoglobulin G (IgG) antibodies as the competitor, was evaluated for potential use in screening for syphilis at various stages. The results obtained by this competitive EIA were compared with those obtained by the fluorescent treponemal antibody absorption (FTA-abs) test and the T. pallidum hemagglutination assay (TPHA). Serum samples from 434 patients with positive TPHA and FTA-abs test results, including patients with primary, latent, secondary, and tertiary syphilis and neurosyphilis, were investigated. Two samples tested negative by competitive EIA but were weakly reactive by the TPHA and the FTA-abs test. Sixteen serum samples from patients with clinically documented active syphilis, including several patients infected with human immunodeficiency virus, tested positive by the competitive EIA. There was a direct inverse correlation between EIA indices and titers in the TPHA and the FTA-abs test for all samples that tested positive. Specificity was assessed by testing 358 serum samples which tested negative for syphilis by TPHA and the FTA-abs test, including 100 serum samples from patients with documented infectious or autoimmune diseases. Only two serum samples gave a weakly positive EIA result. Thus, competitive EIA had a sensitivity of 99.5% and a specificity of 99.4% relative to the results of the FTA-abs test and TPHA. Our evaluation shows that BioElisa Syphilis is a sensitive, specific, and simple assay for screening for syphilis.
Figures
Similar articles
-
Markers of past syphilis in HIV infection comparing Captia Syphilis G anti-treponemal IgG enzyme immunoassay with other treponemal antigen tests.Int J STD AIDS. 1995 Mar-Apr;6(2):101-4. doi: 10.1177/095646249500600207. Int J STD AIDS. 1995. PMID: 7779920
-
Enzyme immunoassay for anti-treponemal IgG: screening or confirmatory test?J Clin Pathol. 1992 Jan;45(1):37-41. doi: 10.1136/jcp.45.1.37. J Clin Pathol. 1992. PMID: 1740512 Free PMC article.
-
Clinical comparison of the Treponema pallidum CAPTIA syphilis-G enzyme immunoassay with the fluorescent treponemal antibody absorption immunoglobulin G assay for syphilis testing.J Clin Microbiol. 1999 Oct;37(10):3233-4. doi: 10.1128/JCM.37.10.3233-3234.1999. J Clin Microbiol. 1999. PMID: 10488183 Free PMC article.
-
Syphilis. Serology.Dermatol Clin. 1998 Oct;16(4):691-8. doi: 10.1016/s0733-8635(05)70034-6. Dermatol Clin. 1998. PMID: 9891668 Review.
-
The fluorescent treponemal antibody-absorption (FTA-ABS) test for syphilis.CRC Crit Rev Clin Lab Sci. 1975 Jan;5(3):315-30. doi: 10.3109/10408367509107046. CRC Crit Rev Clin Lab Sci. 1975. PMID: 1092525 Review.
Cited by
-
Evaluation of SD BIOLINE Syphilis 3.0 for Rapid Diagnosis of Syphilis: Report from a Regional Sexually Transmitted Infection Reference Laboratory in North India.J Lab Physicians. 2016 Jan-Jun;8(1):36-40. doi: 10.4103/0974-2727.176239. J Lab Physicians. 2016. PMID: 27013811 Free PMC article.
-
Treponema pallidum surface immunofluorescence assay for serologic diagnosis of syphilis.Clin Diagn Lab Immunol. 2000 May;7(3):417-21. doi: 10.1128/CDLI.7.3.417-421.2000. Clin Diagn Lab Immunol. 2000. PMID: 10799455 Free PMC article.
-
Comparative evaluation of 15 serological assays for the detection of syphilis infection.Eur J Clin Microbiol Infect Dis. 2007 Oct;26(10):705-13. doi: 10.1007/s10096-007-0346-9. Eur J Clin Microbiol Infect Dis. 2007. PMID: 17647033
-
Clinical utility of a competitive ELISA to detect antibodies against Treponema pallidum.J Clin Lab Anal. 2000;14(2):83-6. doi: 10.1002/(SICI)1098-2825(2000)14:2<83::AID-JCLA8>3.0.CO;2-A. J Clin Lab Anal. 2000. PMID: 10683619 Free PMC article.
-
Comparison of RPR and ELISA with TPHA for the Diagnosis of Syphilis: Implication for Updating Syphilis Point-of-Care Tests in Ethiopia.J Immunol Res. 2018 Jul 8;2018:2978419. doi: 10.1155/2018/2978419. eCollection 2018. J Immunol Res. 2018. PMID: 30069486 Free PMC article.
References
-
- Alfrod C A, Jr, Polt S S, Cassady G E, Straumfjord J V, Remington J S. Gamma-M-fluorescent treponemal antibody in the diagnosis of congenital syphilis. N Engl J Med. 1969;280:1086–1091. - PubMed
-
- Berkowitz K, Buxi L, Fot H E. False negative syphilis screening: the prozone phenomenon, non-immune hychops and diagnosis of syphilis during pregnancy. Am J Obstet Gynecol. 1990;163:975–977. - PubMed
-
- Centers for Disease Control. Progress toward achieving the 1990 objectives for the nation for sexually transmitted disease. Morbid Mortal Weekly Rep. 1990;39:53–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources