Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia In vivo
- PMID: 9454841
- PMCID: PMC2605350
- DOI: 10.1523/JNEUROSCI.18-04-01318.1998
Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia In vivo
Abstract
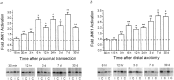
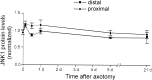
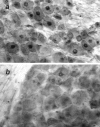
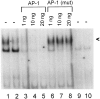
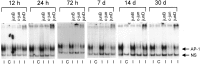
One of the earliest documented molecular events after sciatic nerve injury in adult rats is the rapid, long-term upregulation of the immediate early gene transcription factor c-Jun mRNA and protein in lumbar dorsal root ganglion (DRG) neurons, suggesting that c-Jun may regulate genes that are important both in the early post-injury period and during later peripheral axonal regeneration. However, neither the mechanism through which c-Jun protein is increased nor the level of its post-injury transcriptional activity in axotomized DRGs has been characterized. To determine whether transcriptional activation of c-Jun occurs in response to nerve injury in vivo and is associated with axonal regeneration, we have assayed axotomized adult rat DRGs for evidence of jun kinase activation, c-Jun phosphorylation, and activator protein-1 (AP-1) binding. We report that sciatic nerve transection resulted in chronic activation of c-Jun amino-terminal kinase-1 (JNK) in L4/L5 DRGs concomitant with c-Jun amino-terminal phosphorylation in neurons, and lasting AP-1 binding activity, with both c-Jun and JunD participating in DNA binding complexes. The timing of JNK activation was dependent on the distance of the axotomy site from the DRGs, suggesting the requirement for a retrograde transport-mediated signal. AP-1 binding and c-Jun protein returned to basal levels in DRGs as peripheral regeneration was completed but remained elevated in the case of chronic sprouting, indicating that c-Jun may regulate target genes that are involved in axonal outgrowth.
Figures

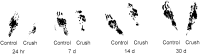
Similar articles
-
Expression of Jun, Fos, and ATF-2 proteins in axotomized explanted and cultured adult rat dorsal root ganglia.Neuroscience. 1998 May;84(1):163-76. doi: 10.1016/s0306-4522(97)00487-9. Neuroscience. 1998. PMID: 9522371
-
Temporal variability of jun family transcription factor levels in peripherally or centrally transected adult rat dorsal root ganglia.Brain Res Mol Brain Res. 1997 Dec 1;52(1):53-61. doi: 10.1016/s0169-328x(97)00211-8. Brain Res Mol Brain Res. 1997. PMID: 9450677
-
The transcription factors c-JUN, JUN D and CREB, but not FOS and KROX-24, are differentially regulated in axotomized neurons following transection of rat sciatic nerve.Brain Res Mol Brain Res. 1992 Jul;14(3):155-65. doi: 10.1016/0169-328x(92)90170-g. Brain Res Mol Brain Res. 1992. PMID: 1331648
-
c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy.Exp Neurol. 1997 Nov;148(1):367-77. doi: 10.1006/exnr.1997.6665. Exp Neurol. 1997. PMID: 9398479
-
Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation.Oncogene. 2002 Sep 19;21(42):6434-45. doi: 10.1038/sj.onc.1205822. Oncogene. 2002. PMID: 12226747
Cited by
-
Role of JNK isoforms in the development of neuropathic pain following sciatic nerve transection in the mouse.Mol Pain. 2012 May 22;8:39. doi: 10.1186/1744-8069-8-39. Mol Pain. 2012. PMID: 22616849 Free PMC article.
-
Opposite regulation of oligodendrocyte apoptosis by JNK3 and Pin1 after spinal cord injury.J Neurosci. 2007 Aug 1;27(31):8395-404. doi: 10.1523/JNEUROSCI.2478-07.2007. J Neurosci. 2007. PMID: 17670986 Free PMC article.
-
Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice.Am J Pathol. 2003 Sep;163(3):833-43. doi: 10.1016/S0002-9440(10)63444-X. Am J Pathol. 2003. PMID: 12937125 Free PMC article.
-
Diminished MTORC1-Dependent JNK Activation Underlies the Neurodevelopmental Defects Associated with Lysosomal Dysfunction.Cell Rep. 2015 Sep 29;12(12):2009-20. doi: 10.1016/j.celrep.2015.08.047. Epub 2015 Sep 17. Cell Rep. 2015. PMID: 26387958 Free PMC article.
-
Can molecular motors drive distance measurements in injured neurons?PLoS Comput Biol. 2009 Aug;5(8):e1000477. doi: 10.1371/journal.pcbi.1000477. Epub 2009 Aug 21. PLoS Comput Biol. 2009. PMID: 19696880 Free PMC article.
References
-
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
-
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. - PubMed
-
- Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. - PubMed
-
- Arvidsson J, Ygge J, Grant G. Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat. Brain Res. 1986;373:15–21. - PubMed
-
- Binetruy B, Smeal T, Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991;351:122–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
