Involvement of ATP-dependent Pseudomonas exotoxin translocation from a late recycling compartment in lymphocyte intoxication procedure
- PMID: 9450963
- PMCID: PMC25269
- DOI: 10.1091/mbc.9.2.387
Involvement of ATP-dependent Pseudomonas exotoxin translocation from a late recycling compartment in lymphocyte intoxication procedure
Abstract
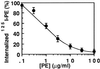
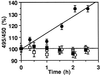
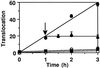
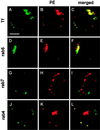
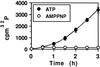
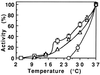
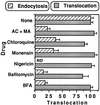
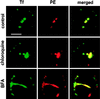
Pseudomonas exotoxin (PE) is a cytotoxin which, after endocytosis, is delivered to the cytosol where it inactivates protein synthesis. Using diaminobenzidine cytochemistry, we found over 94% of internalized PE in transferrin (Tf) -positive endosomes of lymphocytes. When PE translocation was examined in a cell-free assay using purified endocytic vesicles, more than 40% of endosomal 125I-labeled PE was transported after 2 h at 37 degrees C, whereas a toxin inactivated by point mutation in its translocation domain was not translocated. Sorting of endosomes did not allow cell-free PE translocation, whereas active PE transmembrane transport was observed after > 10 min of endocytosis when PE and fluorescent-Tf were localized by confocal immunofluorescence microscopy within a rab5-positive and rab4- and rab7-negative recycling compartment in the pericentriolar region of the cell. Accordingly, when PE delivery to this structure was inhibited using a 20 degrees C endocytosis temperature, subsequent translocation from purified endosomes was impaired. Translocation was also inhibited when endosomes were obtained from cells labeled with PE in the presence of brefeldin A, which caused fusion of translocation-competent recycling endosomes with translocation-incompetent sorting elements. No PE processing was observed in lymphocyte endosomes, the full-sized toxin was translocated and recovered in an enzymatically active form. ATP hydrolysis was found to directly provide the energy required for PE translocation. Inhibitors of endosome acidification (weak bases, protonophores, or bafilomycin A1) when added to the assay did not significantly affect 125I-labeled PE translocation, demonstrating that this transport is independent of the endosome-cytosol pH gradient. Nevertheless, when 125I-labeled PE endocytosis was performed in the presence of one of these molecules, translocation from endosomes was strongly inhibited, indicating that exposure to acidic pH is a prerequisite for PE membrane traversal. When applied during endocytosis, treatments that protect cells against PE intoxication (low temperatures, inhibitors of endosome acidification, and brefeldin A) impaired 125I-labeled PE translocation from purified endosomes. We conclude that PE translocation from a late receptor recycling compartment is implicated in the lymphocyte intoxication procedure.
Figures


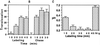

Similar articles
-
Translocation of full-length Pseudomonas exotoxin from endosomes is driven by ATP hydrolysis but requires prior exposure to acidic pH.J Biol Chem. 1996 Oct 18;271(42):26170-3. doi: 10.1074/jbc.271.42.26170. J Biol Chem. 1996. PMID: 8824263
-
[Endosomes and toxin translocation].J Soc Biol. 2001;195(3):235-42. doi: 10.1051/jbio/2001195030235. J Soc Biol. 2001. PMID: 11833460 Review. French.
-
Reduced temperature alters Pseudomonas exotoxin A entry into the mouse LM cell.Infect Immun. 1986 May;52(2):445-53. doi: 10.1128/iai.52.2.445-453.1986. Infect Immun. 1986. PMID: 3699892 Free PMC article.
-
A deletion within the translocation domain of Pseudomonas exotoxin A enhances translocation efficiency and cytotoxicity concomitantly.Mol Microbiol. 1999 Mar;31(5):1385-93. doi: 10.1046/j.1365-2958.1999.01280.x. Mol Microbiol. 1999. PMID: 10200959
-
Redirecting Pseudomonas exotoxin.Semin Cell Biol. 1991 Feb;2(1):31-7. Semin Cell Biol. 1991. PMID: 1954341 Review.
Cited by
-
The apoptogenic toxin AIP56 is a metalloprotease A-B toxin that cleaves NF-κb P65.PLoS Pathog. 2013 Feb;9(2):e1003128. doi: 10.1371/journal.ppat.1003128. Epub 2013 Feb 28. PLoS Pathog. 2013. PMID: 23468618 Free PMC article.
-
Cancer Drug Delivery Systems Using Bacterial Toxin Translocation Mechanisms.Bioengineering (Basel). 2023 Jul 7;10(7):813. doi: 10.3390/bioengineering10070813. Bioengineering (Basel). 2023. PMID: 37508840 Free PMC article. Review.
-
A re-evaluation of the role of histidine-426 within Pseudomonas aeruginosa exotoxin A.Biochem J. 2002 Nov 1;367(Pt 3):601-8. doi: 10.1042/BJ20020768. Biochem J. 2002. PMID: 12160465 Free PMC article.
-
C910 chemical compound inhibits the traffiking of several bacterial AB toxins with cross-protection against influenza virus.iScience. 2022 Jun 6;25(7):104537. doi: 10.1016/j.isci.2022.104537. eCollection 2022 Jul 15. iScience. 2022. PMID: 35769882 Free PMC article.
-
HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses.Mol Biol Cell. 2004 May;15(5):2347-60. doi: 10.1091/mbc.e03-12-0921. Epub 2004 Mar 12. Mol Biol Cell. 2004. PMID: 15020715 Free PMC article.
References
-
- Beaumelle B, Alami M, Hopkins CR. ATP-dependent translocation of ricin across the membrane of purified endosomes. J Biol Chem. 1993;268:23661–23669. - PubMed
-
- Beaumelle B, Bensamar L, Bienvenüe A. Selective translocation of the A chain of Diphtheria Toxin across the membrane of purified endosomes. J Biol Chem. 1992;267:11525–11531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

