Comparison of neurite outgrowth induced by intact and injured sciatic nerves: a confocal and functional analysis
- PMID: 9412511
- PMCID: PMC6793403
- DOI: 10.1523/JNEUROSCI.18-01-00328.1998
Comparison of neurite outgrowth induced by intact and injured sciatic nerves: a confocal and functional analysis
Abstract
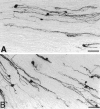
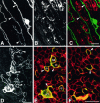
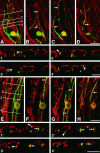
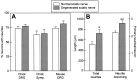
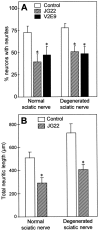
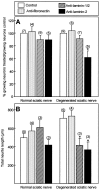
Mechanisms regulating axon growth in the peripheral nervous system have been studied by means of an in vitro bioassay, the tissue section culture, in which regenerating neurons are grown on substrata made up of tissue sections. Sections from intact and degenerated sciatic nerves proved to be different in their ability to support neurite outgrowth of embryonic chick sensory neurons from both qualitative and quantitative points of view. On denervated nerve sections, the total length of neurites elaborated per neuron was almost twice that found on intact nerve sections. In addition, confocal microscopy revealed a striking difference between intact and denervated nerve substrata: on denervated nerve sections, neurites grew inside the internal structures of endoneurial Schwann cell tubes, within the underlying tissue sections, whereas on intact nerve sections neurites extended along endoneurial basal laminae but never entered Schwann cell tubes. Perturbation experiments were used to analyze some of the molecular determinants that control neurite outgrowth in this system. Antibodies directed against the beta1-integrin subunit inhibited neurite extension on both normal and degenerated rat sciatic nerve tissue. Strikingly, however, differential inhibition was observed using antibodies directed against extracellular matrix molecules. Anti-laminin-2 (merosin) antibodies drastically reduced both the percentage of growing neurons and the total length of neurites on denervated nerve sections, but they did not modify these parameters on sections of normal nerve. Taken together, these results suggest that laminin-2/merosin promotes neurite outgrowth in peripheral nerve environments but only after Wallerian degeneration, which is when axons are allowed to extend within endoneurial tubes.
Figures
Similar articles
-
Antibodies directed against the beta 1-integrin subunit and peptides containing the IKVAV sequence of laminin perturb neurite outgrowth of peripheral neurons on immature spinal cord substrata.Neuroscience. 1996 Apr;71(3):773-86. doi: 10.1016/0306-4522(95)00447-5. Neuroscience. 1996. PMID: 8867049
-
Alpha7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons.Mol Cell Neurosci. 2005 Feb;28(2):229-40. doi: 10.1016/j.mcn.2004.08.017. Mol Cell Neurosci. 2005. PMID: 15691705
-
Comparison of the Schwann cell surface and Schwann cell extracellular matrix as promoters of neurite growth.J Neurocytol. 1987 Aug;16(4):539-55. doi: 10.1007/BF01668507. J Neurocytol. 1987. PMID: 3681353
-
In vitro studies on the control of nerve fiber growth by the extracellular matrix of the nervous system.J Physiol (Paris). 1987;82(4):258-70. J Physiol (Paris). 1987. PMID: 3332689 Review.
-
Neurite outgrowth inhibitors in gliotic tissue.Adv Exp Med Biol. 1999;468:207-24. doi: 10.1007/978-1-4615-4685-6_17. Adv Exp Med Biol. 1999. PMID: 10635031 Review.
Cited by
-
Temporal changes in neurotrophic factors and neurite outgrowth in the major pelvic ganglion following cavernous nerve injury.J Neurosci Res. 2015 Jun;93(6):954-63. doi: 10.1002/jnr.23553. Epub 2015 Jan 19. J Neurosci Res. 2015. PMID: 25644064 Free PMC article.
-
Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes.Diabetes. 2009 Dec;58(12):2893-903. doi: 10.2337/db09-0320. Epub 2009 Aug 31. Diabetes. 2009. PMID: 19720799 Free PMC article.
-
Gpr126/Adgrg6 Has Schwann Cell Autonomous and Nonautonomous Functions in Peripheral Nerve Injury and Repair.J Neurosci. 2016 Dec 7;36(49):12351-12367. doi: 10.1523/JNEUROSCI.3854-15.2016. J Neurosci. 2016. PMID: 27927955 Free PMC article.
-
Avian axons undergo Wallerian degeneration after injury and stress.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2016 Nov;202(11):813-822. doi: 10.1007/s00359-016-1123-y. Epub 2016 Sep 10. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2016. PMID: 27614771
-
Alpha4 integrin is expressed during peripheral nerve regeneration and enhances neurite outgrowth.J Neurosci. 2001 Sep 1;21(17):6732-44. doi: 10.1523/JNEUROSCI.21-17-06732.2001. J Neurosci. 2001. PMID: 11517262 Free PMC article.
References
-
- Agius E, Sagot Y, Duprat AM, Cochard P. Antibodies directed against the β1-integrin subunit and peptides containing the IKVAV sequence of laminin perturb neurite outgrowth of peripheral neurons on immature spinal cord substrata. Neuroscience. 1996;71:773–786. - PubMed
-
- Anton ES, Sandrock AJ, Matthew WD. Merosin promotes neurite growth and Schwann cell migration in vitro and nerve regeneration in vivo: evidence using an antibody to merosin, ARM-1. Dev Biol. 1994;164:133–146. - PubMed
-
- Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
