Cytoskeletal association is important for differential targeting of glucose transporter isoforms in Leishmania
- PMID: 9412471
- PMCID: PMC2132635
- DOI: 10.1083/jcb.139.7.1775
Cytoskeletal association is important for differential targeting of glucose transporter isoforms in Leishmania
Abstract
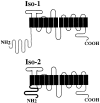
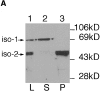
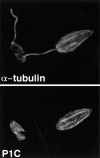
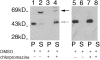
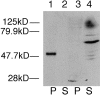
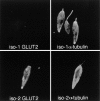
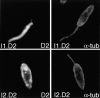
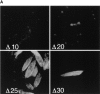
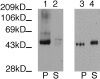
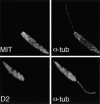
The major glucose transporter of the parasitic protozoan Leishmania enriettii exists in two isoforms, one of which (iso-1) localizes to the flagellar membrane, while the other (iso-2) localizes to the plasma membrane of the cell body, the pellicular membrane. These two isoforms differ only in their cytosolic NH2-terminal domains. Using immunoblots and immunofluorescence microscopy of detergent-extracted cytoskeletons, we have demonstrated that iso-2 associates with the microtubular cytoskeleton that underlies the cell body membrane, whereas the flagellar membrane isoform iso-1 does not associate with the cytoskeleton. Deletion mutants that remove the first 25 or more amino acids from iso-1 are retargeted from the flagellum to the pellicular membrane, suggesting that these deletions remove a signal required for flagellar targeting. Unlike the full-length iso-1 protein, these deletion mutants associate with the cytoskeleton. Our results suggest that cytoskeletal binding serves as an anchor to localize the iso-2 transporter within the pellicular membrane, and that the flagellar targeting signal of iso-1 diverts this transporter into the flagellar membrane and away from the pellicular microtubules.
Figures

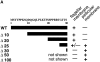
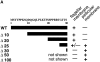

Similar articles
-
Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain.J Cell Biol. 1995 Feb;128(4):499-508. doi: 10.1083/jcb.128.4.499. J Cell Biol. 1995. PMID: 7532172 Free PMC article.
-
Sequences required for the flagellar targeting of an integral membrane protein.Mol Biochem Parasitol. 2004 May;135(1):89-100. doi: 10.1016/j.molbiopara.2004.01.009. Mol Biochem Parasitol. 2004. PMID: 15287590
-
Characterization of a targeting motif for a flagellar membrane protein in Leishmania enriettii.J Biol Chem. 1999 Oct 8;274(41):29543-8. doi: 10.1074/jbc.274.41.29543. J Biol Chem. 1999. PMID: 10506220
-
Evolution of the microtubular cytoskeleton (flagellar apparatus) in parasitic protists.Mol Biochem Parasitol. 2016 Sep-Oct;209(1-2):26-34. doi: 10.1016/j.molbiopara.2016.02.002. Epub 2016 Feb 8. Mol Biochem Parasitol. 2016. PMID: 26868980 Review.
-
Flagellar membrane proteins in kinetoplastid parasites.IUBMB Life. 2015 Sep;67(9):668-76. doi: 10.1002/iub.1411. Epub 2015 Aug 25. IUBMB Life. 2015. PMID: 26599841 Free PMC article. Review.
Cited by
-
Genetic characterization of glucose transporter function in Leishmania mexicana.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3901-6. doi: 10.1073/pnas.0630165100. Epub 2003 Mar 21. Proc Natl Acad Sci U S A. 2003. PMID: 12651954 Free PMC article.
-
Secretory pathway of trypanosomatid parasites.Microbiol Mol Biol Rev. 2002 Mar;66(1):122-54; table of contents. doi: 10.1128/MMBR.66.1.122-154.2002. Microbiol Mol Biol Rev. 2002. PMID: 11875130 Free PMC article. Review.
-
Both sequence and context are important for flagellar targeting of a glucose transporter.J Cell Sci. 2012 Jul 15;125(Pt 14):3293-8. doi: 10.1242/jcs.103028. Epub 2012 Mar 30. J Cell Sci. 2012. PMID: 22467850 Free PMC article.
-
Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein.J Biol Chem. 2011 Sep 23;286(38):33109-17. doi: 10.1074/jbc.M111.240895. Epub 2011 Jul 22. J Biol Chem. 2011. PMID: 21784841 Free PMC article.
-
Lysosomal degradation of Leishmania hexose and inositol transporters is regulated in a stage-, nutrient- and ubiquitin-dependent manner.Int J Parasitol. 2011 Jun;41(7):791-800. doi: 10.1016/j.ijpara.2011.02.003. Epub 2011 Apr 9. Int J Parasitol. 2011. PMID: 21447343 Free PMC article.
References
-
- Balber AE. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit Rev Immunol. 1990;10:177–201. - PubMed
-
- Baranski TJ, Koelsch G, Hartsuck JA, Kornfeld S. Mapping and molecular modeling of a recognition domain for lysosomal enzyme targeting. J Biol Chem. 1991;266:23365–23372. - PubMed
-
- Bennett V, Stenbuck PJ. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979;280:468–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

