Enhanced immunogenicity of HIV-1 vaccine construct by modification of the native peptide sequence
- PMID: 9380724
- PMCID: PMC23507
- DOI: 10.1073/pnas.94.20.10856
Enhanced immunogenicity of HIV-1 vaccine construct by modification of the native peptide sequence
Abstract
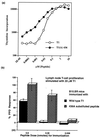
Viral proteins are not naturally selected for high affinity major histocompatibility complex (MHC) binding sequences; indeed, if there is any selection, it is likely to be negative in nature. Thus, one should be able to increase viral peptide binding to MHC in the rational design of synthetic peptide vaccines. The T1 helper peptide from the HIV-1 envelope protein was made more immunogenic for inducing T cell proliferation to the native sequence by replacing a residue that exerts an adverse influence on peptide binding to an MHC class II molecule. Mice immunized with vaccine constructs combining the more potent Th helper (Th) epitope with a cytotoxic T lymphocyte (CTL) determinant developed greatly enhanced CTL responses. Use of class II MHC-congenic mice confirmed that the enhancement of CTL response was due to class II-restricted help. Thus, enhanced T cell help is key for optimal induction of CTL, and, by modification of the native immunogen to increase binding to MHC, it is possible to develop second generation vaccine constructs that enhance both Th cell activation and CTL induction.
Figures



Similar articles
-
Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs.J Immunol. 1994 Jan 15;152(2):549-56. J Immunol. 1994. PMID: 8283036
-
Progress toward an artificial vaccine for HIV: identification of helper and cytotoxic T-cell epitopes and methods of immunization.Biotechnol Ther. 1991;2(1-2):123-35. Biotechnol Ther. 1991. PMID: 1726961 Review.
-
Antigenicity of HIV-derived T helper determinants in the context of carrier recombinant proteins: effect on T helper cell repertoire selection.Eur J Immunol. 1996 Oct;26(10):2461-9. doi: 10.1002/eji.1830261029. Eur J Immunol. 1996. PMID: 8898961
-
Designing and engineering of DNA-vaccine construction encoding multiple CTL-epitopes of major HIV-1 antigens.Vaccine. 2004 Apr 16;22(13-14):1672-82. doi: 10.1016/j.vaccine.2003.09.048. Vaccine. 2004. PMID: 15068850
-
Peptide component vaccine engineering: targeting the AIDS virus.Int Rev Immunol. 1990;7(1):85-107. doi: 10.3109/08830189009061767. Int Rev Immunol. 1990. PMID: 1722499 Review.
Cited by
-
Antibodies to the CD4-binding site of HIV-1 gp120 suppress gp120-specific CD4 T cell response while enhancing antibody response.Infect Agent Cancer. 2008 Jul 18;3:11. doi: 10.1186/1750-9378-3-11. Infect Agent Cancer. 2008. PMID: 18638381 Free PMC article.
-
Progress on new vaccine strategies against chronic viral infections.J Clin Invest. 2004 Aug;114(4):450-62. doi: 10.1172/JCI22674. J Clin Invest. 2004. PMID: 15314679 Free PMC article. Review.
-
TARP vaccination is associated with slowing in PSA velocity and decreasing tumor growth rates in patients with Stage D0 prostate cancer.Oncoimmunology. 2016 Jul 1;5(8):e1197459. doi: 10.1080/2162402X.2016.1197459. eCollection 2016 Aug. Oncoimmunology. 2016. PMID: 27622067 Free PMC article.
-
Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions.J Clin Invest. 1998 Sep 15;102(6):1239-48. doi: 10.1172/JCI3714. J Clin Invest. 1998. PMID: 9739058 Free PMC article.
-
A push-pull vaccine strategy using Toll-like receptor ligands, IL-15, and blockade of negative regulation to improve the quality and quantity of T cell immune responses.Vaccine. 2012 Jun 19;30(29):4323-7. doi: 10.1016/j.vaccine.2011.11.034. Epub 2011 Nov 21. Vaccine. 2012. PMID: 22115635 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

