Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members
- PMID: 9356461
- PMCID: PMC24966
- DOI: 10.1073/pnas.94.23.12401
Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members
Abstract
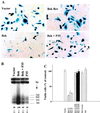
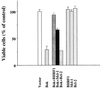
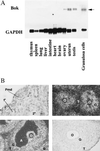
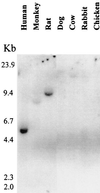
In the intracellular death program, hetero- and homodimerization of different anti- and pro-apoptotic Bcl-2-related proteins are critical in the determination of cell fate. From a rat ovarian fusion cDNA library, we isolated a new pro-apoptotic Bcl-2 gene, Bcl-2-related ovarian killer (Bok). Bok had conserved Bcl-2 homology (BH) domains 1, 2, and 3 and a C-terminal transmembrane region present in other Bcl-2 proteins, but lacked the BH4 domain found only in anti-apoptotic Bcl-2 proteins. In the yeast two-hybrid system, Bok interacted strongly with some (Mcl-1, BHRF1, and Bfl-1) but not other (Bcl-2, Bcl-xL, and Bcl-w) anti-apoptotic members. This finding is in direct contrast to the ability of other pro-apoptotic members (Bax, Bak, and Bik) to interact with all of the anti-apoptotic proteins. In addition, negligible interaction was found between Bok and different pro-apoptotic members. In mammalian cells, overexpression of Bok induced apoptosis that was blocked by the baculoviral-derived cysteine protease inhibitor P35. Cell killing induced by Bok was also suppressed following coexpression with Mcl-1 and BHRF1 but not with Bcl-2, further indicating that Bok heterodimerized only with selective anti-apoptotic Bcl-2 proteins. Northern blot analysis indicated that Bok was highly expressed in the ovary, testis and uterus. In situ hybridization analysis localized Bok mRNA in granulosa cells, the cell type that underwent apoptosis during follicle atresia. Identification of Bok as a new pro-apoptotic Bcl-2 protein with restricted tissue distribution and heterodimerization properties could facilitate elucidation of apoptosis mechanisms in reproductive tissues undergoing hormone-regulated cyclic cell turnover.
Figures


Similar articles
-
BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members.Mol Endocrinol. 1998 Sep;12(9):1432-40. doi: 10.1210/mend.12.9.0166. Mol Endocrinol. 1998. PMID: 9731710
-
A splicing variant of the Bcl-2 member Bok with a truncated BH3 domain induces apoptosis but does not dimerize with antiapoptotic Bcl-2 proteins in vitro.J Biol Chem. 1998 Nov 13;273(46):30139-46. doi: 10.1074/jbc.273.46.30139. J Biol Chem. 1998. PMID: 9804769
-
Characterization of the antiapoptotic Bcl-2 family member myeloid cell leukemia-1 (Mcl-1) and the stimulation of its message by gonadotropins in the rat ovary.Endocrinology. 1999 Dec;140(12):5469-77. doi: 10.1210/endo.140.12.7171. Endocrinology. 1999. PMID: 10579309
-
Structural biology of the Bcl-2 family of proteins.Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review.
-
Tissue-specific Bcl-2 protein partners in apoptosis: An ovarian paradigm.Physiol Rev. 2000 Apr;80(2):593-614. doi: 10.1152/physrev.2000.80.2.593. Physiol Rev. 2000. PMID: 10747202 Review.
Cited by
-
The role of BCL-2 family proteins in regulating apoptosis and cancer therapy.Front Oncol. 2022 Oct 12;12:985363. doi: 10.3389/fonc.2022.985363. eCollection 2022. Front Oncol. 2022. PMID: 36313628 Free PMC article. Review.
-
BOK controls ER proteostasis and physiological ER stress responses in neurons.Front Cell Dev Biol. 2022 Aug 15;10:915065. doi: 10.3389/fcell.2022.915065. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36060797 Free PMC article.
-
The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage.J Biol Chem. 2013 Aug 30;288(35):25340-25349. doi: 10.1074/jbc.M113.496570. Epub 2013 Jul 24. J Biol Chem. 2013. PMID: 23884412 Free PMC article.
-
Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP.Mol Cell Biol. 2005 Apr;25(8):3117-26. doi: 10.1128/MCB.25.8.3117-3126.2005. Mol Cell Biol. 2005. PMID: 15798198 Free PMC article.
-
Bok, Bcl-2-related Ovarian Killer, Is Cell Cycle-regulated and Sensitizes to Stress-induced Apoptosis.J Biol Chem. 2006 Aug 11;281(32):22729-35. doi: 10.1074/jbc.M604705200. Epub 2006 Jun 13. J Biol Chem. 2006. PMID: 16772296 Free PMC article.
References
-
- Thompson C B. Science. 1995;267:1456–1462. - PubMed
-
- Steller H. Science. 1995;267:1445–1449. - PubMed
-
- Kroemer G. Nat Med. 1997;3:614–620. - PubMed
-
- Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Cell. 1993;75:653–660. - PubMed
-
- Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

