Activating mutations for the met tyrosine kinase receptor in human cancer
- PMID: 9326629
- PMCID: PMC23495
- DOI: 10.1073/pnas.94.21.11445
Activating mutations for the met tyrosine kinase receptor in human cancer
Abstract
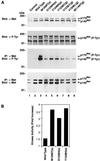
Recently, mutations in the Met tyrosine kinase receptor have been identified in both hereditary and sporadic forms of papillary renal carcinoma. We have introduced the corresponding mutations into the met cDNA and examined the effect of each mutation in biochemical and biological assays. We find that the Met mutants exhibit increased levels of tyrosine phosphorylation and enhanced kinase activity toward an exogenous substrate when compared with wild-type Met. Moreover, NIH 3T3 cells expressing mutant Met molecules form foci in vitro and are tumorigenic in nude mice. Enzymatic and biological differences were evident among the various mutants examined, and the somatic mutations were generally more active than those of germ-line origin. A strong correlation between the enzymatic and biological activity of the mutants was observed, indicating that tumorigenesis by Met is quantitatively related to its level of activation. These results demonstrate that the Met mutants originally identified in human papillary renal carcinoma are oncogenic and thus are likely to play a determinant role in this disease, and these results raise the possibility that activating Met mutations also may contribute to other human malignancies.
Figures


Similar articles
-
Novel mutations of the MET proto-oncogene in papillary renal carcinomas.Oncogene. 1999 Apr 8;18(14):2343-50. doi: 10.1038/sj.onc.1202547. Oncogene. 1999. PMID: 10327054
-
Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas.Nat Genet. 1997 May;16(1):68-73. doi: 10.1038/ng0597-68. Nat Genet. 1997. PMID: 9140397
-
Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene.Cancer Res. 1998 Apr 15;58(8):1719-22. Cancer Res. 1998. PMID: 9563489
-
Papillary renal cell carcinoma: analysis of germline mutations in the MET proto-oncogene in a clinic-based population.Genet Test. 2001 Summer;5(2):101-6. doi: 10.1089/109065701753145547. Genet Test. 2001. PMID: 11551094 Review.
-
The role of non-ras transforming genes in chemical carcinogenesis.Environ Health Perspect. 1991 Jun;93:33-40. doi: 10.1289/ehp.919333. Environ Health Perspect. 1991. PMID: 1685444 Free PMC article. Review.
Cited by
-
Detection and therapeutic implications of c-Met mutations in small cell lung cancer and neuroendocrine tumors.Curr Pharm Des. 2013;19(5):833-40. Curr Pharm Des. 2013. PMID: 22973954 Free PMC article.
-
A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma.Eur Urol. 2012 Dec;62(6):1013-9. doi: 10.1016/j.eururo.2012.06.043. Epub 2012 Jun 27. Eur Urol. 2012. PMID: 22771265 Free PMC article. Clinical Trial.
-
Structural characterization of autoinhibited c-Met kinase produced by coexpression in bacteria with phosphatase.Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3563-8. doi: 10.1073/pnas.0600048103. Epub 2006 Feb 28. Proc Natl Acad Sci U S A. 2006. PMID: 16537444 Free PMC article.
-
Ex vivo and in vivo biological effects of a truncated form of the receptor tyrosine kinase stk when activated by interaction with the friend spleen focus-forming virus envelope glycoprotein or by point mutation.J Virol. 2004 May;78(9):4573-81. doi: 10.1128/jvi.78.9.4573-4581.2004. J Virol. 2004. PMID: 15078939 Free PMC article.
-
Engineering, Characterization, and Biological Evaluation of an Antibody Targeting the HGF Receptor.Front Immunol. 2021 Dec 3;12:775151. doi: 10.3389/fimmu.2021.775151. eCollection 2021. Front Immunol. 2021. PMID: 34925346 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

