Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major
- PMID: 9314562
- PMCID: PMC2199076
- DOI: 10.1084/jem.186.7.1137
Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major
Abstract
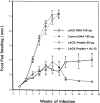
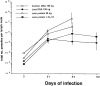
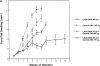
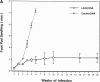
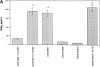
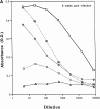
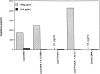
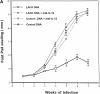
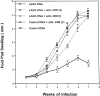
To determine whether DNA immunization could elicit protective immunity to Leishmania major in susceptible BALB/c mice, cDNA for the cloned Leishmania antigen LACK was inserted into a euykaryotic expression vector downstream to the cytomegalovirus promoter. Susceptible BALB/c mice were then vaccinated subcutaneously with LACK DNA and challenged with L. major promastigotes. We compared the protective efficacy of LACK DNA vaccination with that of recombinant LACK protein in the presence or absence of recombinant interleukin (rIL)-12 protein. Protection induced by LACK DNA was similar to that achieved by LACK protein and rIL-12, but superior to LACK protein without rIL-12. The immunity conferred by LACK DNA was durable insofar as mice challenged 5 wk after vaccination were still protected, and the infection was controlled for at least 20 wk after challenge. In addition, the ability of mice to control infection at sites distant to the site of vaccination suggests that systemic protection was achieved by LACK DNA vaccination. The control of disease progression and parasitic burden in mice vaccinated with LACK DNA was associated with enhancement of antigen-specific interferon-gamma (IFN-gamma) production. Moreover, both the enhancement of IFN-gamma production and the protective immune response induced by LACK DNA vaccination was IL-12 dependent. Unexpectedly, depletion of CD8(+) T cells at the time of vaccination or infection also abolished the protective response induced by LACK DNA vaccination, suggesting a role for CD8(+) T cells in DNA vaccine induced protection to L. major. Thus, DNA immunization may offer an attractive alternative vaccination strategy against intracellular pathogens, as compared with conventional vaccination with antigens combined with adjuvants.
Figures





Similar articles
-
The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge.J Immunol. 2001 Apr 15;166(8):5122-8. doi: 10.4049/jimmunol.166.8.5122. J Immunol. 2001. PMID: 11290794
-
Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis.Vaccine. 2004 Sep 28;22(29-30):3865-76. doi: 10.1016/j.vaccine.2004.04.015. Vaccine. 2004. PMID: 15364433
-
Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells.J Immunol. 2000 Jul 15;165(2):915-24. doi: 10.4049/jimmunol.165.2.915. J Immunol. 2000. PMID: 10878366
-
Leishmaniasis: current status of vaccine development.Curr Mol Med. 2004 Sep;4(6):667-79. doi: 10.2174/1566524043360203. Curr Mol Med. 2004. PMID: 15357215 Review.
-
New insight into the mechanisms underlying Th2 cell development and susceptibility to Leishmania major in BALB/c mice.Microbes Infect. 1999 Jan;1(1):59-64. doi: 10.1016/s1286-4579(99)80015-x. Microbes Infect. 1999. PMID: 10847767 Review. No abstract available.
Cited by
-
Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis.Infect Immun. 2001 Aug;69(8):4719-25. doi: 10.1128/IAI.69.8.4719-4725.2001. Infect Immun. 2001. PMID: 11447143 Free PMC article.
-
Anti-Leishmanial Vaccines: Assumptions, Approaches, and Annulments.Vaccines (Basel). 2019 Oct 18;7(4):156. doi: 10.3390/vaccines7040156. Vaccines (Basel). 2019. PMID: 31635276 Free PMC article. Review.
-
Heterologous priming-boosting with DNA and modified vaccinia virus Ankara expressing tryparedoxin peroxidase promotes long-term memory against Leishmania major in susceptible BALB/c Mice.Infect Immun. 2007 Feb;75(2):852-60. doi: 10.1128/IAI.01490-06. Epub 2006 Nov 13. Infect Immun. 2007. PMID: 17101647 Free PMC article.
-
The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates.Pathogens. 2022 Apr 2;11(4):431. doi: 10.3390/pathogens11040431. Pathogens. 2022. PMID: 35456106 Free PMC article. Review.
-
Intramuscular immunization with p36(LACK) DNA vaccine induces IFN-gamma production but does not protect BALB/c mice against Leishmania chagasi intravenous challenge.Parasitol Res. 2005 Dec;98(1):67-74. doi: 10.1007/s00436-005-0008-8. Epub 2005 Nov 1. Parasitol Res. 2005. PMID: 16261353
References
-
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, De Witt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science (Wash DC) 1993;259:1745–1749. - PubMed
-
- Xiang ZQ, Spitalnik S, Tran M, Wunner WH, Cheng J, Ertl HC. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

