Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants
- PMID: 9279175
- PMCID: PMC1720665
- DOI: 10.1136/fn.77.1.f4
Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants
Abstract
Aims: To improve energy intake in sick very low birthweight (VLBW) infants; to decrease growth problems, lessen pulmonary morbidity, shorten hospital stay, and avoid possible feeding related morbidity. Morbidity in VLBW infants thought to be associated with parenteral and enteral feeding includes bronchopulmonary dysplasia, necrotising enterocolitis, septicaemia, cholestasis and osteopenia of prematurity.
Methods: A prospective randomised controlled trial (RCT) comparing two types of nutritional intervention was performed involving 125 sick VLBW infants in the setting of a regional neonatal intensive care unit. Babies were randomly allocated to either an aggressive nutritional regimen (group A) or a control group (group B). Babies in group B received a conservative nutritional regimen while group A received a package of more aggressive parenteral and enteral nutrition. Statistical analysis was done using Student's t test, the Mann-Whitney U test, the chi 2 test and logistic regression.
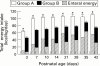
Results: There was an excess of sicker babies in group A, as measured by initial disease severity (P < 0.01), but mean total energy intakes were significantly higher (P < 0.001) in group A at days 3 to 42 while receiving total or partial parenteral nutrition. Survival and the incidences of bronchopulmonary dysplasia, septicaemia, cholestasis, osteopenia and necrotising enterocolitis were similar in both groups. Growth in early life and at discharge from hospital was significantly better in babies in group A. There were no decreases in pulmonary morbidity or hospital stay.
Conclusion: Nutritional intake in sick VLBW infants can be improved without increasing the risk of adverse clinical or metabolic sequelae. Improved nutritional intake resulted in better growth, both in discharge, but did not decrease pulmonary morbidity or shorten hospital stay.
Figures

Similar articles
-
Calorie intake in sick versus respiratory stable very low birthweight babies.Acta Paediatr Jpn. 1996 Oct;38(5):449-54. doi: 10.1111/j.1442-200x.1996.tb03525.x. Acta Paediatr Jpn. 1996. PMID: 8942002
-
Trophic feedings for parenterally fed infants.Cochrane Database Syst Rev. 2005 Jul 20;(3):CD000504. doi: 10.1002/14651858.CD000504.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2009 Jul 08;(3):CD000504. doi: 10.1002/14651858.CD000504.pub3. PMID: 16034854 Updated. Review.
-
Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier.Pediatrics. 2004 Dec;114(6):e699-706. doi: 10.1542/peds.2004-0911. Epub 2004 Nov 15. Pediatrics. 2004. PMID: 15545616 Clinical Trial.
-
A standardized nutrition approach for very low birth weight neonates improves outcomes, reduces cost and is not associated with increased rates of necrotizing enterocolitis, sepsis or mortality.J Perinatol. 2013 Nov;33(11):851-7. doi: 10.1038/jp.2013.66. Epub 2013 Jun 13. J Perinatol. 2013. PMID: 23765172
-
Early trophic feeding for very low birth weight infants.Cochrane Database Syst Rev. 2009 Jul 8;(3):CD000504. doi: 10.1002/14651858.CD000504.pub3. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2013 Mar 28;(3):CD000504. doi: 10.1002/14651858.CD000504.pub4. PMID: 19588318 Updated. Review.
Cited by
-
Effects of food on physical and sleep complaints in children with ADHD: a randomised controlled pilot study.Eur J Pediatr. 2010 Sep;169(9):1129-38. doi: 10.1007/s00431-010-1196-5. Epub 2010 Apr 17. Eur J Pediatr. 2010. PMID: 20401617 Free PMC article. Clinical Trial.
-
Risk factors and correlates of neonatal growth velocity in extremely low gestational age newborns: the ELGAN Study.Neonatology. 2013;104(4):298-304. doi: 10.1159/000351020. Epub 2013 Oct 30. Neonatology. 2013. PMID: 24192897 Free PMC article.
-
Lipid emulsions for parenterally fed preterm infants.Cochrane Database Syst Rev. 2019 Jun 4;6(6):CD013163. doi: 10.1002/14651858.CD013163.pub2. Cochrane Database Syst Rev. 2019. PMID: 31158919 Free PMC article.
-
Prematurity and programming: contribution of neonatal Intensive Care Unit interventions.J Dev Orig Health Dis. 2013 Apr;4(2):121-33. doi: 10.1017/S204017441200061X. J Dev Orig Health Dis. 2013. PMID: 25054678 Free PMC article.
-
Can We Understand the Pathobiology of Bronchopulmonary Dysplasia?J Pediatr. 2017 Nov;190:27-37. doi: 10.1016/j.jpeds.2017.08.041. J Pediatr. 2017. PMID: 29144252 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
