A calcium responsive element that regulates expression of two calcium binding proteins in Purkinje cells
- PMID: 9238065
- PMCID: PMC23159
- DOI: 10.1073/pnas.94.16.8842
A calcium responsive element that regulates expression of two calcium binding proteins in Purkinje cells
Abstract
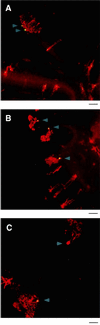
Calbindin D28 encodes a calcium binding protein that is expressed in the cerebellum exclusively in Purkinje cells. We have used biolistic transfection of organotypic slices of P12 cerebellum to identify a 40-bp element from the calbindin promoter that is necessary and sufficient for Purkinje cell specific expression in this transient in situ assay. This element (PCE1) is also present in the calmodulin II promoter, which regulates expression of a second Purkinje cell Ca2+ binding protein. Expression of high levels of exogenous calbindin or calretinin decreased transcription mediated by PCE1 in Purkinje cells 2.5- to 3-fold, whereas the presence of 1 microM ionomycin in the extracellular medium increased expression. These results demonstrate that PCE1 is a component of a cell-specific and Ca2+-sensitive transcriptional regulatory mechanism that may play a key role in setting the Ca2+ buffering capacity of Purkinje cells.
Figures

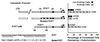
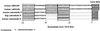
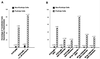
Similar articles
-
Transient expression of calretinin during development of chick cerebellum. Comparison with calbindin-D28k.Neurosci Res. 1993 Jun;17(1):53-61. doi: 10.1016/0168-0102(93)90029-p. Neurosci Res. 1993. PMID: 8414217
-
Presence of calbindin D28K and GAD67 mRNAs in both orthotopic and ectopic Purkinje cells of staggerer mice suggests that staggerer acts after the onset of cytodifferentiation.J Neurosci Res. 1996 May 1;44(3):255-62. doi: 10.1002/(SICI)1097-4547(19960501)44:3<255::AID-JNR6>3.0.CO;2-F. J Neurosci Res. 1996. PMID: 8723764
-
Beta 1 isoform-specific regulation of a triiodothyronine-induced gene during cerebellar development.Mol Endocrinol. 1992 Nov;6(11):1874-80. doi: 10.1210/mend.6.11.1282672. Mol Endocrinol. 1992. PMID: 1282672
-
[Structure, expression and control of calbindins-D].Ann Endocrinol (Paris). 1990;51(3-4):108-11. Ann Endocrinol (Paris). 1990. PMID: 2291623 Review. French.
-
Structure of the human cDNAs and genes coding for calbindin D28K and calretinin.Adv Exp Med Biol. 1990;269:27-34. doi: 10.1007/978-1-4684-5754-4_4. Adv Exp Med Biol. 1990. PMID: 2191560 Review. No abstract available.
Cited by
-
Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport.EMBO J. 2006 Jul 12;25(13):2978-88. doi: 10.1038/sj.emboj.7601186. Epub 2006 Jun 8. EMBO J. 2006. PMID: 16763551 Free PMC article.
-
Time course of SERCA 2b and calreticulin expression in Purkinje neurons of ethanol-fed rats with behavioral correlates.Alcohol Alcohol. 2013 Nov-Dec;48(6):667-78. doi: 10.1093/alcalc/agt062. Epub 2013 Jul 24. Alcohol Alcohol. 2013. PMID: 23884168 Free PMC article.
-
The lurcher mutation and ionotropic glutamate receptors: contributions to programmed neuronal death in vivo.Brain Pathol. 1998 Oct;8(4):795-807. doi: 10.1111/j.1750-3639.1998.tb00201.x. Brain Pathol. 1998. PMID: 9804384 Free PMC article. Review.
-
Nanodomains of single Ca2+ channels contribute to action potential repolarization in cortical neurons.J Neurosci. 2007 Jan 17;27(3):483-95. doi: 10.1523/JNEUROSCI.3816-06.2007. J Neurosci. 2007. PMID: 17234581 Free PMC article.
-
Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4281-6. doi: 10.1073/pnas.0737363100. Epub 2003 Mar 14. Proc Natl Acad Sci U S A. 2003. PMID: 12640146 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

