Molecular cloning of a peroxisomal Ca2+-dependent member of the mitochondrial carrier superfamily
- PMID: 9238007
- PMCID: PMC22978
- DOI: 10.1073/pnas.94.16.8509
Molecular cloning of a peroxisomal Ca2+-dependent member of the mitochondrial carrier superfamily
Abstract
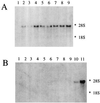
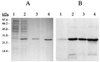
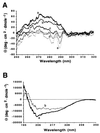
A cDNA from a novel Ca2+-dependent member of the mitochondrial solute carrier superfamily was isolated from a rabbit small intestinal cDNA library. The full-length cDNA clone was 3,298 nt long and coded for a protein of 475 amino acids, with four elongation factor-hand motifs located in the N-terminal half of the molecule. The 25-kDa N-terminal polypeptide was expressed in Escherichia coli, and it was demonstrated that it bound Ca2+, undergoing a reversible and specific conformational change as a result. The conformation of the polypeptide was sensitive to Ca2+ which was bound with high affinity (Kd approximately 0.37 microM), the apparent Hill coefficient for Ca2+-induced changes being about 2.0. The deduced amino acid sequence of the C-terminal half of the molecule revealed 78% homology to Grave disease carrier protein and 67% homology to human ADP/ATP translocase; this sequence homology identified the protein as a new member of the mitochondrial transporter superfamily. Northern blot analysis revealed the presence of a single transcript of about 3,500 bases, and low expression of the transporter could be detected in the kidney but none in the liver. The main site of expression was the colon with smaller amounts found in the small intestine proximal to the ileum. Immunoelectron microscopy localized the transporter in the peroxisome, although a minor fraction was found in the mitochondria. The Ca2+ binding N-terminal half of the transporter faces the cytosol.
Figures
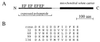

Similar articles
-
A novel mitochondrial Ca2+-dependent solute carrier in the liver identified by mRNA differential display.J Biol Chem. 2003 Mar 14;278(11):9520-7. doi: 10.1074/jbc.m208398200. J Biol Chem. 2003. PMID: 12645546
-
Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain.J Biol Chem. 1998 Sep 4;273(36):23327-34. doi: 10.1074/jbc.273.36.23327. J Biol Chem. 1998. PMID: 9722566
-
Identification of 30 kDa calsequestrin-binding protein, which regulates calcium release from sarcoplasmic reticulum of rabbit skeletal muscle.Biochem J. 1998 Nov 1;335 ( Pt 3)(Pt 3):541-7. doi: 10.1042/bj3350541. Biochem J. 1998. PMID: 9794793 Free PMC article.
-
PMP47, a peroxisomal homologue of mitochondrial solute carrier proteins.Trends Biochem Sci. 1993 Nov;18(11):427-8. Trends Biochem Sci. 1993. PMID: 8291088 Review. No abstract available.
-
The 70 kDa peroxisomal membrane protein: an ATP-binding cassette transporter protein involved in peroxisome biogenesis.Semin Cell Biol. 1993 Feb;4(1):45-52. doi: 10.1006/scel.1993.1006. Semin Cell Biol. 1993. PMID: 8453064 Review.
Cited by
-
The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP.Biochem J. 2000 Jan 15;345 Pt 2(Pt 2):161-79. Biochem J. 2000. PMID: 10620491 Free PMC article. Review.
-
Presence of a member of the mitochondrial carrier family in hydrogenosomes: conservation of membrane-targeting pathways between hydrogenosomes and mitochondria.Mol Cell Biol. 2000 Apr;20(7):2488-97. doi: 10.1128/MCB.20.7.2488-2497.2000. Mol Cell Biol. 2000. PMID: 10713172 Free PMC article.
-
Calcium in peroxisomes: An essential messenger in an essential cell organelle.Front Cell Dev Biol. 2022 Aug 30;10:992235. doi: 10.3389/fcell.2022.992235. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36111338 Free PMC article. Review.
-
The peroxisome: an update on mysteries.Histochem Cell Biol. 2012 May;137(5):547-74. doi: 10.1007/s00418-012-0941-4. Epub 2012 Mar 14. Histochem Cell Biol. 2012. PMID: 22415027 Review.
-
Disruption of actin filaments induces mitochondrial Ca2+ release to the cytoplasm and [Ca2+]c changes in Arabidopsis root hairs.BMC Plant Biol. 2010 Mar 24;10:53. doi: 10.1186/1471-2229-10-53. BMC Plant Biol. 2010. PMID: 20334630 Free PMC article.
References
-
- Dickerson R E. Nature (London) 1980;283:210–212. - PubMed
-
- De Duve C. Sci Am. 1983;248:52–62. - PubMed
-
- Lazarow P B, Fujiki Y. Annu Rev Cell Biol. 1985;1:489–530. - PubMed
-
- Schatz G. J Biol Chem. 1996;271:31763–31766. - PubMed
-
- Cuezva J M, Flores A I, Liras A, Santarén J F, Alconada A. Biol Cell. 1993;77:47–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

