A metastable intermediate state of microtubule dynamic instability that differs significantly between plus and minus ends
- PMID: 9214385
- PMCID: PMC2139954
- DOI: 10.1083/jcb.138.1.105
A metastable intermediate state of microtubule dynamic instability that differs significantly between plus and minus ends
Abstract
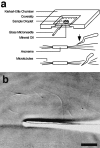
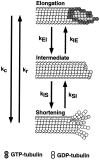
The current two-state GTP cap model of microtubule dynamic instability proposes that a terminal crown of GTP-tubulin stabilizes the microtubule lattice and promotes elongation while loss of this GTP-tubulin cap converts the microtubule end to shortening. However, when this model was directly tested by using a UV microbeam to sever axoneme-nucleated microtubules and thereby remove the microtubule's GTP cap, severed plus ends rapidly shortened, but severed minus ends immediately resumed elongation (Walker, R.A., S. Inoué, and E.D. Salmon. 1989. J. Cell Biol. 108: 931-937). To determine if these previous results were dependent on the use of axonemes as seeds or were due to UV damage, or if they instead indicate an intermediate state in cap dynamics, we performed UV cutting of self-assembled microtubules and mechanical cutting of axoneme-nucleated microtubules. These independent methods yielded results consistent with the original work: a significant percentage of severed minus ends are stable after cutting. In additional experiments, we found that the stability of both severed plus and minus ends could be increased by increasing the free tubulin concentration, the solution GTP concentration, or by assembling microtubules with guanylyl-(alpha,beta)-methylene-diphosphonate (GMPCPP). Our results show that stability of severed ends, particularly minus ends, is not an artifact, but instead reveals the existence of a metastable kinetic intermediate state between the elongation and shortening states of dynamic instability. The kinetic properties of this intermediate state differ between plus and minus ends. We propose a three-state conformational cap model of dynamic instability, which has three structural states and four transition rate constants, and which uses the asymmetry of the tubulin heterodimer to explain many of the differences in dynamic instability at plus and minus ends.
Figures



Similar articles
-
Asymmetric behavior of severed microtubule ends after ultraviolet-microbeam irradiation of individual microtubules in vitro.J Cell Biol. 1989 Mar;108(3):931-7. doi: 10.1083/jcb.108.3.931. J Cell Biol. 1989. PMID: 2921286 Free PMC article.
-
Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate.J Cell Biol. 1991 Jul;114(1):73-81. doi: 10.1083/jcb.114.1.73. J Cell Biol. 1991. PMID: 2050742 Free PMC article.
-
How tubulin subunits are lost from the shortening ends of microtubules.J Struct Biol. 1997 Mar;118(2):107-18. doi: 10.1006/jsbi.1997.3844. J Struct Biol. 1997. PMID: 9126637 Review.
-
Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies.J Cell Biol. 1988 Oct;107(4):1437-48. doi: 10.1083/jcb.107.4.1437. J Cell Biol. 1988. PMID: 3170635 Free PMC article.
-
Analysis of dynamic instability of steady-state microtubules in vitro by video-enhanced differential interference contrast microscopy with an appendix by Emin Oroudjev.Methods Cell Biol. 2010;95:189-206. doi: 10.1016/S0091-679X(10)95011-5. Methods Cell Biol. 2010. PMID: 20466136 Review.
Cited by
-
Microtubule plus-end dynamics in Xenopus egg extract spindles.Mol Biol Cell. 2004 Apr;15(4):1776-84. doi: 10.1091/mbc.e03-11-0824. Epub 2004 Feb 6. Mol Biol Cell. 2004. PMID: 14767058 Free PMC article.
-
Three-dimensional microtubule behavior in Xenopus egg extracts reveals four dynamic states and state-dependent elastic properties.Biophys J. 2008 Aug;95(3):1474-86. doi: 10.1529/biophysj.107.128223. Epub 2008 Apr 25. Biophys J. 2008. PMID: 18441022 Free PMC article.
-
With the Permission of Microtubules: An Updated Overview on Microtubule Function During Axon Pathfinding.Front Mol Neurosci. 2021 Dec 2;14:759404. doi: 10.3389/fnmol.2021.759404. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34924953 Free PMC article. Review.
-
Identification of XMAP215 as a microtubule-destabilizing factor in Xenopus egg extract by biochemical purification.J Cell Biol. 2003 Apr 28;161(2):349-58. doi: 10.1083/jcb.200211095. J Cell Biol. 2003. PMID: 12719474 Free PMC article.
-
Yeast kinetochores do not stabilize Stu2p-dependent spindle microtubule dynamics.Mol Biol Cell. 2003 Oct;14(10):4181-95. doi: 10.1091/mbc.e03-03-0180. Epub 2003 Jul 25. Mol Biol Cell. 2003. PMID: 14517328 Free PMC article.
References
-
- Bell CW, Fraser C, Sale WS, Tang W-JY, Gibbons IR. Preparation and purification of dynein. Methods Cell Biol. 1982;24:373–397. - PubMed
-
- Billger MA, Bhatacharjee G, Williams RC., Jr Dynamic instability of microtubules assembled from microtubule-associated-protein-free tubulin: neither variability of growth and shortening rates nor “rescue” requires microtubule-associated proteins. Biochemistry. 1996;35:13656–13663. - PubMed
-
- Caplow M. Microtubule dynamics. Curr Opin Cell Biol. 1992;4:58–65. - PubMed
-
- Caplow M, Shanks J. Induction of microtubule catastrophe by formation of tubulin-GDP and apotubulin subunits at microtubule ends. Biochemistry. 1995;34:15732–15741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

