Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells
- PMID: 9182660
- PMCID: PMC2132535
- DOI: 10.1083/jcb.137.6.1255
Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells
Abstract
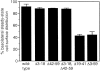
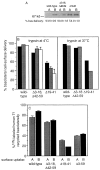
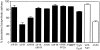
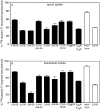
In polarized Madin-Darby canine kidney (MDCK) cells, the transferrin receptor (TR) is selectively delivered to the basolateral surface, where it internalizes transferrin via clathrin-coated pits and recycles back to the basolateral border. Mutant tailless receptors are sorted randomly in both the biosynthetic and endocytic pathways, indicating that the basolateral sorting of TR is dependent upon a signal located within the 61-amino acid cytoplasmic domain. To identify the basolateral sorting signal of TR, we have analyzed a series of mutant human TR expressed in MDCK cells. We find that residues 19-41 are sufficient for basolateral sorting from both the biosynthetic and endocytic pathways and that this is the only region of the TR cytoplasmic tail containing basolateral sorting information. The basolateral sorting signal is distinct from the YTRF internalization signal contained within this region and is not tyrosine based. Detailed functional analyses of the mutant TR indicate that residues 29-35 are the most important for basolateral sorting from the biosynthetic pathway. The structural requirements for basolateral sorting of internalized receptors from the endocytic pathway are not identical. The most striking difference is that alteration of G31DNS34 to YTRF impairs basolateral sorting of newly synthesized receptors from the biosynthetic pathway but not internalized receptors from the endocytic pathway. Also, mutations have been identified that selectively impair basolateral sorting of internalized TRs from the endocytic pathway without affecting basolateral sorting of newly synthesized receptors. These results imply that there are subtle differences in the recognition of the TR basolateral sorting signal by separate sorting machinery located within the biosynthetic and endocytic pathways.
Figures

Similar articles
-
Signal-dependent trafficking of beta-amyloid precursor protein-transferrin receptor chimeras in madin-darby canine kidney cells.J Biol Chem. 1998 Feb 6;273(6):3732-9. doi: 10.1074/jbc.273.6.3732. J Biol Chem. 1998. PMID: 9452505
-
Structural requirements for major histocompatibility complex class II invariant chain trafficking in polarized Madin-Darby canine kidney cells.J Biol Chem. 1997 May 2;272(18):11757-62. doi: 10.1074/jbc.272.18.11757. J Biol Chem. 1997. PMID: 9115230
-
Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment.J Cell Biol. 1994 Jul;126(2):317-30. doi: 10.1083/jcb.126.2.317. J Cell Biol. 1994. PMID: 8034737 Free PMC article.
-
Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism.J Cell Biol. 1996 Oct;135(1):139-52. doi: 10.1083/jcb.135.1.139. J Cell Biol. 1996. PMID: 8858169 Free PMC article.
-
Molecular sorting in polarized and non-polarized cells: common problems, common solutions.J Cell Sci Suppl. 1993;17:1-7. doi: 10.1242/jcs.1993.supplement_17.1. J Cell Sci Suppl. 1993. PMID: 8144683 Review.
Cited by
-
Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association.J Virol. 2000 Jul;74(14):6538-45. doi: 10.1128/jvi.74.14.6538-6545.2000. J Virol. 2000. PMID: 10864667 Free PMC article.
-
Galectin-4-mediated transcytosis of transferrin receptor.J Cell Sci. 2014 Oct 15;127(Pt 20):4457-69. doi: 10.1242/jcs.153437. Epub 2014 Sep 1. J Cell Sci. 2014. PMID: 25179596 Free PMC article.
-
Lipid domain structure of the plasma membrane revealed by patching of membrane components.J Cell Biol. 1998 May 18;141(4):929-42. doi: 10.1083/jcb.141.4.929. J Cell Biol. 1998. PMID: 9585412 Free PMC article.
-
Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor.Mol Biol Cell. 2004 Apr;15(4):1746-59. doi: 10.1091/mbc.e03-11-0832. Epub 2004 Feb 6. Mol Biol Cell. 2004. PMID: 14767057 Free PMC article.
-
Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia.Traffic. 2011 Apr;12(4):483-98. doi: 10.1111/j.1600-0854.2010.01155.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199217 Free PMC article.
References
-
- Bates P, Young JAT, Varmus HE. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. - PubMed
-
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science (Wash DC) 1989;245:1499–1501. - PubMed

